Minimally invasive esophagectomy for esophageal squamous cell carcinoma—Shanghai Chest Hospital experience
Esophageal cancer is a common gastrointestinal cancer and ranks sixth in tumor-related death. Esophageal adenocarcinoma is prevalence in European countries and the United States, while esophageal squamous cell carcinoma is frequently observed in Asian countries (1). Radical surgical resection combined with systematic lymph node dissection has always been used as a significant approach for treating esophageal cancer. In recent years, preoperative neoadjuvant therapy has significantly improved the long-term outcomes of the surgical treatment of esophageal cancer (2,3). Conventional open surgical procedures for esophageal cancer are very traumatic and lead to a high incidence of postoperative complications, especially pulmonary complications, as well as high postoperative mortality, despite continuous advances in perioperative management and surgical techniques. Since Cuschieri et al. (4) first reported thoracoscopic surgery in minimally invasive esophagectomy (MIE) in 1992, MIE has become a standard surgical approach for esophageal cancer in the world (5-7). It was approved that due to significantly reduced surgical trauma, MIE lowers postoperative morbidity and mortality while achieving a tumor resection efficacy comparable with the conventional open esophagectomy (6,8-10).
In recent years, the Department of Esophageal Surgery of Shanghai Chest Hospital has been committed to the promotion and practice of minimally invasive techniques, and from the conventional thoraco-laparoscopic assisted surgery to the Da Vinci robot assisted esophageal resection, MIE has gradually become a routine surgical treatment of resectable esophageal cancer. In this report, we summarize the past MIE experiences performed at the Shanghai Chest Hospital and analyze their progress in early postoperative recovery and effectiveness in achieving satisfactory oncological outcomes.
Preoperative assessment of patients
All patients underwent preoperative staging and evaluation, including enhanced computed tomography (CT) scan of the chest and abdomen, neck ultrasound, upper gastrointestinal endoscopy under ordinary white light, and endoscopy coupled with endoscopic ultrasound (EUS), as well as bronchoscopy for patients with lesions above the carina. Fluoroxyglucose-18 positron emission computed tomography (PET) was only used on those with regional or distant metastases that were difficult to determine. Patients with multiple lymph node metastases and over stage T3 development were subjected to preoperative induction therapy. All patients received complete two-field lymph node dissection, including those along the bilateral recurrent laryngeal nerve. As long as the imaging assessment indicated it was surgically resectable, it was regarded as an indication for minimally invasive esophageal surgery.
Choice of surgical approach
Whether conventional esophagectomy or MIE, the approach for esophagus resection has been rather controversial. Open esophagectomy typically use the transhiatal approach and the transthoracic approach, the latter including the Ivor Lewis, McKeown, and Sweet procedures. Each surgical option has certain advantages. Currently, randomized controlled studies on the advantages and disadvantages of various surgical choices are lacking. Previously, Hulscher and his colleagues reported a large, randomized comparative study with 220 patients conducted in the Netherlands in which some had undergone transthoracic esophagectomy (TTE) and some had undergone transhiatal esophagectomy (THE) (11) and found that the patients who received THE had a shorter operation time, less blood loss, and lower incidence of postoperative complications, but showed no difference in perioperative mortality. However, the patients who received TTE had a higher number of lymph nodes dissected. The 5-year disease-free survival and the overall survival of the TTE group demonstrated improvement but were not statistically significant. Although the open TTE exhibited better survival tendencies and better lymph node dissection, its incidence of postoperative complications was higher than that of THE. In theory, transthoracic MIE can achieve the same oncological surgical outcome while lowering the incidence of postoperative complications. Relative to the high incidence of adenocarcinoma in the lower esophageal segment and the esophagogastric junction in European countries and the United States, esophageal cancer in China are dominated by squamous cell carcinoma, with lesions mostly in the middle and the lower esophageal segments. In our center, esophageal cancer in the middle and the lower esophageal segments accounted for 88.1% of the total cases. Different pathological types and different tumor location require different surgical strategies for the treatment of esophageal cancer. In China, MIE mostly adopts the Ivor Lewis or the McKeown procedure (12-14). A review study revealed that compared with the McKeown procedure, the Ivor Lewis procedure shows a lower incidence of intraoperative damage of the recurrent laryngeal nerve, shorter postoperative hospital stay, and less intraoperative blood loss, but a similar incidence of anastomotic leakage (15). However, this study lacked pathological and long-term follow-up results, and a high proportion of the cases were esophageal adenocarcinoma, it only has limited implications to the treatment of esophageal cancer in China.
In the Shanghai Chest Hospital, the McKeown procedure was mainly adopted; among 207 MIE procedures performed, 193 were completed via the McKeown procedure, accounting for 93.2% of the total cases.
Anesthesia and surgical posture
General anesthesia and anesthesia ventilation via single-lumen endotracheal tube was routinely performed. A “single-lumen endotracheal tube + CO2 artificial pneumothorax” technique was adopted for intraoperative lung collapse. The advantage of this technique is that after the establishment of artificial pneumothorax, the lung collapse is more rapid and complete, the gap between the adipose tissue in the mediastinum is widened, and so is the gap between tissues peripheral to lymph nodes, which makes it easier to separate them while reducing blood loss. The pressure of artificial pneumothorax is generally 6–8 mmHg, exerting little effect on hemodynamics. In addition, the application of a single-lumen endotracheal tube is also conducive to the surgical resection of paratracheal tissue.
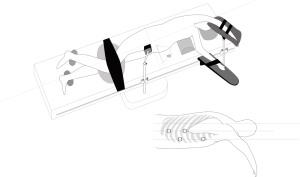
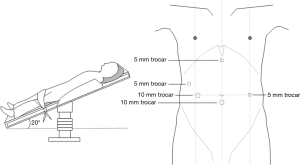
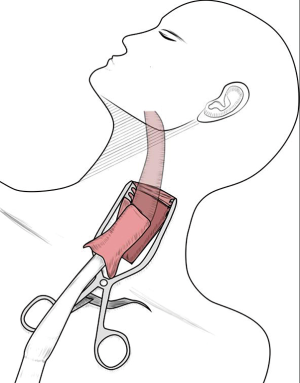
Two postures, the left lateral decubitus position and the prone position, were usually adopted for the chest operation. Due to anatomical similarities, the left lateral decubitus position was adopted in the initial applications of MIE. Luketich et al. (16) reported that the lateral decubitus position was chosen for more than 1,000 minimally invasive surgical operations for esophageal cancer. At the same time, the prone position exposes longitudinal organs and structures more clearly and has been gradually introduced in MIE and widely practiced (8,17,18). In a review study, Markar et al. (19) showed that compared with that in a lateral position, MIE performed with the patient in a prone position could reduce the incidence of postoperative pulmonary complications and intraoperative blood loss while increasing the number of mediastinal lymph nodes dissected. Two other studies (18,20) also showed that using a prone position for MIE could significantly improve postoperative oxygen delivery, reduce the incidence of pulmonary complications, and facilitate early postoperative recovery. However, one of the major drawbacks of the prone position is the need to change position when switching to thoracotomy during an emergency. In the Shanghai Chest Hospital, patients were in the left lateral recumbent position and leaned forward 30° with artificial pneumothorax, which not only provides a clear surgical area but also avoids posture change in the case of emergency. At present, this position has also been accepted by many hospitals in the world (Figure 1). In the abdominal laparoscopy-assisted operation, the surgical position is to tilt the patient with the head raised and the feet lowered, with the left side raised 30 degrees, which is conducive to the downward and rightward movements of the omentum and colon and a better exposure of the splenic portal, facilitating the separation of short gastric vessels (Figure 2).
Surgical procedure
Thoracic procedure
The patient assumed the left lateral position, leaning forward 30 degrees to establish CO2 artificial pneumothorax (pressured at approximately 6–8 mmHg), and the surgeon stood on the ventral side of the patient. The seventh intercostal of the right anterior axillary line was set as the laparoendoscopic observation hole, the third intercostal was set as the primary operation hole, the sixth intercostal of the right midaxillary line was set as the secondary operation hole, and the ninth intercostal of the right midaxillary line was set as the operation hole for the assistant surgeon. In addition, a purse string suture was punctured through the third intercostal, located between the posterior edge of the scapula and the spine, to make it possible to pull the esophagus during the operation (Figure 1).
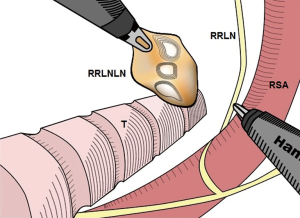
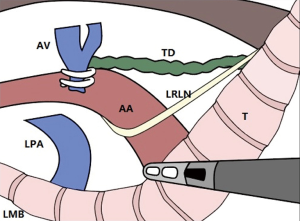
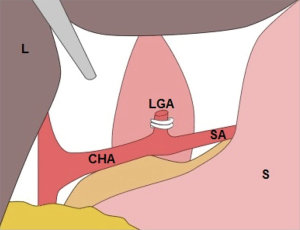
First, the mediastinal pleura anterior and posterior to the esophagus was longitudinally incised above the azygos vein with an electrocautery hook, and the incision was advanced along the right side of the vagus nerve until reaching the upper edge of the right supraclavicular artery. After carefully exposing the right recurrent laryngeal nerve, the soft tissues and lymph nodes from the rear of the nerve, in front of the esophagus, and up to the bottom of the thyroid were completely dissected (Figure 3). Then, the azygous vein was detached, the mediastinal pleura were incised at the anterior edge of the azygos vein, and the soft tissue in front of the aorta was pushed toward the esophagus and dissected. In the case of stage T3 or above, the azygos vein and the aortic thoracic duct were also dissected. The middle and lower esophagus and the surrounding soft tissues, together with the subcarinal lymphatic nodes, were completely dissected. After the medial esophagus was properly separated from the mediastinum in an upward direction, the esophagus was pulled to the right and back with a pulling wire. The left recurrent laryngeal nerve lymph nodes were dissected from the left mediastinal pleura and the recurrent laryngeal nerve (Figure 4). The thoracic parabronchial lymph nodes and the lymph nodes inferior to the aortic arch were dissected along the external wall of the left main bronchus. At the end of the chest surgery, one 28-Fr chest tube and two mediastinal drainage tubes were installed.
Abdominal procedure
The patient assumed a supine position, with the head raised and the feet lowered, at approximately a 20-degree angle. CO2 artificial pneumothorax was established (at a pressure of approximately 14 mmHg). Five abdominal Traco units were used, and a Trocar (camera, 10 mm) was placed 1 cm below the umbilicus. One operation hole was set at the xiphoid to make it easier to pull the liver and stomach, a second operation hole for the ultrasonic surgical knife was set between the mid clavicular line and the umbilicus, a third operation hole was set between the anterior axillary line and the midaxillary line, and the operation hole for the assistant surgeon was set at the umbilicus level of the upper left abdomen (Figure 2). After being dissociated via skeletalization, the left gastric artery was disarticulated by clipping with a HemoLock clip (Figure 5). The lymph nodes in the area from the para-cardia to the left gastric artery were dissected. After retaining the right gastroepiploic artery that was disarticulated from the stomach, a longitudinal midline abdominal incision of approximately 5–8 cm in length was made from under the xiphoid, the stomach was removed of the body, and tubular gastroplasty was performed and raised to the neck through the posterior sternum or esophageal bed. The pyloric sphincter was disassociated through conventional pressing to facilitate postoperative emptying. A nasogastric tube and duodenal feeding tube were arranged.
Neck operation
An oblique incision approximately 6 cm in length was made along the left anterior edge of the sternocleidomastoid muscle to expose and mobilize the cervical esophagus, and the paraesophageal lymph nodes were dissected. In the case of upper segment esophageal cancer, routine bilateral neck lymph node dissection was performed. Esophagogastric anastomosis or manual esophagogastric anastomosis on the neck was performed with a circular stapler device (Figure 6). After placing the drainage flap, the neck was closed.
Postoperative treatment and follow-up
Postoperative patients were routinely fasted and given intravenous nutrition, and enteral nutrition was initiated on the second day after the operation. The anteroposterior and lateral chest radiographs were examined on the third day after the operation, when the chest drainage tube and gastric tube were removed as appropriate. The mediastinal drainage tubes were removed 7–10 days after the operation, and the patient was discharged and continued on enteral nutrition support. Two weeks after discharge from the hospital, the patient was scheduled for a follow-up examination in the hospital to perform upper gastrointestinal radiography using iodine solution to confirm anastomotic healing. If no abnormalities were found, the duodenal feeding tube was removed, and the patient began a fluid diet and gradually increased to a semifluid diet.
The patient was scheduled with regular postoperative outpatient patient follow-ups once every 3 months in the first year after surgery, then once every 6 months until the fifth year after surgery. Follow-up items include chest CT, neck ultrasound, upper gastrointestinal radiography, tumor marker blood tests (every 6 months), and esophagoscopy (annually).
Perioperative outcomes
The application of minimally invasive surgery and the improvement in surgical details have resulted in improved perioperative outcomes. Several studies have shown that the application of MIE reduces intraoperative bleeding and the incidence of perioperative complications, especially pulmonary complications, and shortens postoperative hospital stay and intensive care unit (ICU) stay lengths. Luketich et al. (5) reported clinical data that retrospectively analyzed the surgical MIE treatment of 222 cases and found that the postoperative ICU stay and postoperative hospital stay lengths were 1 and 7 days, respectively, and the perioperative mortality rate, the incidence of postoperative anastomotic leakage, and the incidence of postoperative pneumonia were 1.4%, 11.7%, and 7.7%, respectively, which demonstrated advantages compared with those of esophagectomy on esophageal cancer of the same stage. Subsequently, randomized controlled studies have been conducted to compare the efficacies of MIE and open esophageal surgery. In 2012, Biere et al. (6) conducted a multicenter randomized controlled study that included 56 cases of open esophagectomy and 59 cases of MIE to compare the incidence of postoperative complications. The results showed that the incidence of postoperative pulmonary complications was significantly lower in the MIE group (12%) than in the open esophagectomy group (34%).
In the Shanghai Chest Hospital, the incidence of postoperative pneumonia was 13.5%, which is close to that reported in previous studies. Another meta-analysis (21) came to a similar conclusion that MIE reduces the incidence of postoperative pulmonary complications and intraoperative bleeding. However, in terms of other postoperative complications such as anastomotic leakage or stenosis, recurrent laryngeal nerve palsy, and perioperative mortality, the two groups exhibited no differences.
In addition to the incidence of perioperative complications, the surgery quality evaluation includes surgical oncology indicators, e.g., lymph node dissection efficiency and radical treatment outcome of the tumor site. In a multicenter randomized controlled study conducted in the Netherlands that compared the efficacies of MIE and open esophagectomy, it was found that the R0 resection rates of the MIE and open esophagectomy groups were 92% and 84%, respectively (P=0.08), the average numbers of lymph nodes dissected were 20 and 21, respectively, and the difference was statistically insignificant (6). Luketich was involved in another multicenter Stage II clinical trial that included 110 patients and purported to investigate the feasibility of MIE for esophageal cancer; it was found that the R0 resection rate was 96.1%, and the average number of lymph nodes dissected was 19 (10). Thirunavukarasu et al. (22) analyzed the 2010–2012 patient data from the US National Cancer Database and showed that among 4,047 patients with esophagectomy, of which 997 were performed with MIE, the incidence of a positive surgical margin in the MIE and open esophagectomy groups was 8.1% and 7.4%, respectively, differing insignificantly; however, the lymph node dissection outcome of the MIE group was superior to that of the open esophagectomy group. Therefore, MIE achieves a tumor radical treatment outcome similar to that of open esophagectomy, but a better outcome in terms of lymph node dissection.
In our study, the R0 tumor resection rate was 95.9%, which is similar to that of previous studies. Thanks to the clear field of view of the laparoscope, after the initial learning curve, MIE is able to achieve stable lymph node dissection efficiency, especially in the resection of the bilateral lymph nodes of the recurrent laryngeal nerve chain, which plays a key role in the lymphatic metastasis of advanced esophageal squamous cell carcinoma. In our study, the average number of lymph nodes dissected was 12, and the lymph node sampling rates of the left and right recurrent laryngeal nerve were 55.4% and 74.1%, respectively.
Long-term survival results
MIE has been widely accepted by the majority of thoracic surgeons due to its better perioperative recovery but is not yet accepted by some who are doubtful of its long-term effects. Currently, randomized controlled trials assessing the long-term effects of MIE are rare. A recently published multi-center randomized controlled trial by the TIME team (23) compared the long-term follow-up results of 56 open esophagectomy cases and 59 MIE cases and showed no statistically significant differences in 3-year overall survival and disease-free survival between the two groups. Although it was a multi-center study, it only included a small number of cases that were pathologically dominated by adenocarcinoma, so more studies on the long-term efficacy of MIE in squamous cell carcinoma are needed. In another meta-analysis on 1,549 patients (21), the results showed that MIE achieved a 5-year survival comparable to that of open esophagectomy while showing certain superiority in terms of 2-year survival. Yerokun et al. (24) used data from the US National Cancer Database and the propensity scores to analyze the long-term outcomes of MIE and open esophagectomy. The results showed that regardless of squamous or adenocarcinoma, the 3-year survival rates of the two groups exhibited no statistically significant differences, and the 3-year overall survival rates of the MIE and open esophagectomy groups of squamous cell carcinoma were 54.7% and 56.3%, respectively.
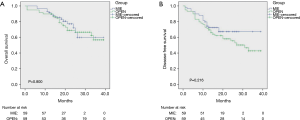
In the Shanghai Chest Hospital, the 3- and 5-year overall survival was 73.6% and 60.7%, respectively, which shows significant improvement in the long-term survival of esophageal cancer treatment compared with previous studies. Our previous study (25) indicated that for locally advanced stage T3 esophageal cancer, MIE was able to achieve a mid- and long-term outcome comparable to that of open esophagectomy (Figure 7).
Conclusions
MIE is a safe and feasible method for treating esophageal cancer, and after overcoming the learning curve, surgeons who are skilled in open esophagectomy are also able to master this minimally invasive technique. By reducing the incidence of postoperative complications, especially pulmonary complications, MIE can improve the postoperative quality of life of patients with esophageal cancer and achieve long-term survival outcomes comparable to those of traditional open esophagectomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524-48. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68-74. [Crossref] [PubMed]
- Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb 1992;37:7-11. [PubMed]
- Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg 2003;238:486-94; discussion 494-85.
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Kawahara K, Maekawa T, Okabayashi K, et al. Video-assisted thoracoscopic esophagectomy for esophageal cancer. Surg Endosc 1999;13:218-23. [Crossref] [PubMed]
- Wang H, Shen Y, Feng M, et al. Outcomes, quality of life, and survival after esophagectomy for squamous cell carcinoma: A propensity score-matched comparison of operative approaches. J Thorac Cardiovasc Surg 2015;149:1006-14; discussion 1014-5.e4.
- Takeuchi H, Miyata H, Ozawa S, et al. Comparison of Short-Term Outcomes Between Open and Minimally Invasive Esophagectomy for Esophageal Cancer Using a Nationwide Database in Japan. Ann Surg Oncol 2017;24:1821-7. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Franchetti Y, et al. Minimally invasive esophagectomy: results of a prospective phase II multicenter trial-the eastern cooperative oncology group (E2202) study. Ann Surg 2015;261:702-7. [Crossref] [PubMed]
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [Crossref] [PubMed]
- Li KK, Wang YJ, Liu XH, et al. The effect of postoperative complications on survival of patients after minimally invasive esophagectomy for esophageal cancer. Surg Endosc 2017;31:3475-82. [Crossref] [PubMed]
- Lin M, Shen Y, Feng M, et al. Minimally invasive esophagectomy: Chinese experiences. J Vis Surg 2016;2:134. [Crossref] [PubMed]
- Ye B, Zhong CX, Yang Y, et al. Lymph node dissection in esophageal carcinoma: Minimally invasive esophagectomy vs open surgery. World J Gastroenterol 2016;22:4750-6. [Crossref] [PubMed]
- van Workum F, Berkelmans GH, Klarenbeek BR, et al. McKeown or Ivor Lewis totally minimally invasive esophagectomy for cancer of the esophagus and gastroesophageal junction: systematic review and meta-analysis. J Thorac Dis 2017;9:S826-S833. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Palanivelu C, Prakash A, Senthilkumar R, et al. Minimally invasive esophagectomy: thoracoscopic mobilization of the esophagus and mediastinal lymphadenectomy in prone position--experience of 130 patients. J Am Coll Surg 2006;203:7-16. [Crossref] [PubMed]
- Tanaka E, Okabe H, Kinjo Y, et al. Advantages of the prone position for minimally invasive esophagectomy in comparison to the left decubitus position: better oxygenation after minimally invasive esophagectomy. Surg Today 2015;45:819-25. [Crossref] [PubMed]
- Markar SR, Wiggins T, Antonowicz S, et al. Minimally invasive esophagectomy: Lateral decubitus vs. prone positioning; systematic review and pooled analysis. Surg Oncol 2015;24:212-9. [Crossref] [PubMed]
- Otsubo D, Nakamura T, Yamamoto M, et al. Prone position in thoracoscopic esophagectomy improves postoperative oxygenation and reduces pulmonary complications. Surg Endosc 2017;31:1136-41. [Crossref] [PubMed]
- Guo W, Ma X, Yang S, et al. Combined thoracoscopic-laparoscopic esophagectomy versus open esophagectomy: a meta-analysis of outcomes. Surg Endosc 2016;30:3873-81. [Crossref] [PubMed]
- Thirunavukarasu P, Gabriel E, Attwood K, et al. Nationwide analysis of short-term surgical outcomes of minimally invasive esophagectomy for malignancy. Int J Surg 2016;25:69-75. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Yerokun BA, Sun Z, Yang CJ, et al. Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Population-Based Analysis. Ann Thorac Surg 2016;102:416-23. [Crossref] [PubMed]
- Zhang Xb, Yang Y, Ye B, et al. Minimally invasive esophagectomy is a safe surgical treatment for locally advanced pathologic T3 esophageal squamous cell carcinoma. J Thorac Dis 2017;9:2982-91. [Crossref] [PubMed]


