Benchmark values for transthoracic esophagectomy are not set as the defined “best possible”—a validation study
Introduction
Quality of surgical care is a current topic in the management of esophageal cancer (1), which causes significant disease burden and cancer-related mortality worldwide (2). Although survival rates have improved with modern multimodal treatment protocols, surgery still offers the best chance for cure in early or locally advanced disease (3,4). Esophagectomy is the mainstay of these protocols. Nevertheless, it remains a complex and cost-demanding procedure, with high postoperative morbidity and mortality (5,6). In order to reduce surgery-associated morbidity and mortality, minimally invasive esophagectomy (MIE) has been introduced (7). Single center series and one randomized study suggested reduced postoperative morbidity, shorter hospital stay, and improved patient satisfaction after MIE (8-11). In a population-based study from Finland and Sweden, MIE was associated with lower 90-day mortality (12).
In 2009, worldwide cancer collaboration database results were published consisting of 7,884 patients who underwent open esophagectomy, with a 30-day mortality of 2% and a 1-year survival rate of 78% (13). Just recently, 13 high-volume centers published the outcomes of a cohort of 1,057 MIEs, with a 30-day mortality of 2.1%, 90-day mortality of 5.2%, and 1-year survival rate of 82.3% (1). Outcomes of esophagectomy vary significantly, however, between centers. The top 10% of hospitals in the US report a 90-day mortality of 2.2% compared to 16.2% reported by the bottom 10% (5). To monitor performance in surgery, benchmarking has gained considerable weight (14). Recently, benchmark values for MIE were defined from the results of “ideal” patients with low comorbidity operated on at 13 high-volume centers (1). In this study by Schmidt et al., benchmark values were defined as the 75th percentile of the median proportions, giving the “best possible” results for MIE to be pursued.
The aim of this study was to evaluate the validity of the defined benchmark parameters by comparing them to the outcomes in a medium-volume center performing around 20 MIEs annually.
Methods
Patients
The MIE program at Central Finland Central Hospital, an affiliated hospital, was started by an experienced surgeon (ES) in September 2012 (15). During the past years, after a gradual increase in the number of operations, the annual caseload has been 18 to 20 operations. From September 2012 to November 2017, all cancer operations of the tubular esophagus or cancer at the esophagogastric junction (n=79) were performed using minimally invasive techniques. Three patients who underwent MIE due to a benign indication (intractable stricture, unfunctional esophagus after surgery for perforation, and treatment resistant gastroesophageal reflux disease after four fundoplications) were included in the analysis, for a total of 82 patients.
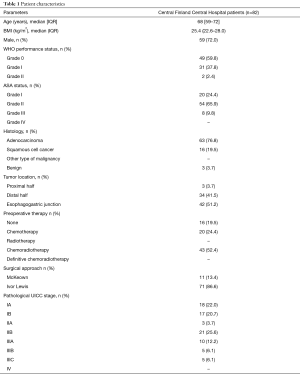
In cancer patients, the preoperative diagnostic protocol included endoscopy, endoscopic ultrasound, body computed tomography (CT), and positron emission tomography (PET)-CT. Also, patients’ exercise tolerance and nutritional status was thoroughly evaluated (16). The patients’ baseline information is provided in Table 1. Of 79 patients, 63 received neoadjuvant therapy, including either chemotherapy or chemoradiation. The intended chemotherapy cycle consisted of a single dose of epirubicin (50 mg/m2) and cisplatin (60 mg/m2), and 5-fluorouracil 200 mg/m2/day for 21 days. Three cycles were given preoperatively and three postoperatively. Chemoradiotherapy included paclitaxel (50 mg/m2) and carboplatin (180–300 mg) for four cycles, and 23 fractions of radiation for a total of 41.4 Gy. Patients were restaged before surgery with either CT or PET-CT according to primary fluorodeoxyglucose (FDG) avidity of the tumor. Only high FDG-avid tumors were re-staged by PET-CT. The operation was performed after a 6-week recovery period.
Full table
Operative approach
A minimally invasive Ivor Lewis procedure with intrathoracic anastomosis was our preferred procedure (n=71). Neck anastomosis (McKeown) was performed in 11 patients. In 75 patients, the planned operation was transthoracic total MIE and in 7 patients a hybrid procedure, either with chest (n=2) or abdomen (n=5), was performed using a minimally invasive technique. The reasons for the planned thoracotomies were T3-tumor location against the left main bronchus. Of 75 patients, two were converted to a hybrid procedure due to a short gastric conduit after a previous fundoplication or severe adhesions in the abdomen after peritonitis. All patients with malignant conditions underwent en bloc lymphadenectomy. For surgery, 3-dimensional optics has been used since June 2013. The extent of lymphadenectomy from our center has been previously described (17). Laparoscopy was performed in supine and thoracoscopy in left lateral position. Intrathoracic end-to-side anastomosis was performed using a circular stapler and was reinforced with an omental flap modified from a technique described by Luketich (Figure 1) (18). Surgery included a feeding jejunostomy tube and endoscopic pyloric dilatation in all patients. All the operations were performed by an experienced thoracic and esophageal surgeon (ES) together with a general surgeon with expertise in upper GI surgery in a standardized manner (19-21).
Perioperative treatment and follow-up
All patients were admitted a day before the planned surgery. Approximately 12 hours prior to surgery, they received 40 mg of subcutaneous enoxaparin. Pneumatic compression socks were placed for surgery and worn until mobilization. Early mobilization and chest physiotherapy was started at the intensive care unit (ICU) on the day of surgery. On postoperative day two, enteral feeding was started. Gradual oral feeding was permitted on the fifth postoperative day after an esophageal contrast study done by CT. The aim was to discharge patients on postoperative day 9. Follow up for 5 years was planned for all patients after surgery. The median follow-up time was 22 months. In three patients, follow-up ended before reaching the 90-day mark. Mortality data was confirmed from the nationwide and obligatory Cause of Death registry held by Statistics Finland. The end of follow-up for this study was December 18, 2017.
Benchmarking
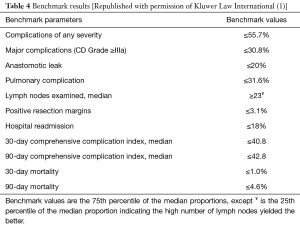
Benchmark values were directly obtained from the study by Schmidt et al. (1). As suggested by the authors, primary outcome measures for comparison were overall and major (Clavien-Dindo ≥3a) (22) morbidity, readmissions, anastomotic, and pulmonary complications; all at 30 days after hospital discharge. The complications basic platform published by the Esophagectomy Complications Consensus Group (ECCG) (23) was used in a similar way as the benchmark study. Positive resection margin, the number of examined lymph nodes, 30- and 90-day comprehensive complications index (24), and 30- and 90-day mortality rates were reported.
Pathological analysis
Paraffin-embedded esophageal samples were analyzed by a gastrointestinal pathologist according to the normal standardized protocol. Staging was performed according to the American Joint Committee on Cancer, seventh edition criteria (4,17).
The study was approved by the Central Finland Hospital district. Because of the retrospective nature of the study, patient informed consent or ethical statement was not required.
Results
Basic characteristics of study patients
The median age of patients treated at our center was 68 (IQR, 59–72) years, with a male majority (72.0%). Most patients were diagnosed with esophageal adenocarcinoma (n=63, 76.8%). The majority of malignant tumors were located in the distal esophagus or at the esophagogastric junction (n=76, 96.2%), with Sievert type II tumor being the most prevalent type (n=40). Of 79 cancer patients, 63 (79.7%) received either neoadjuvant chemotherapy or chemoradiotherapy due to locally advanced disease. Complete response or near complete response was observed 21 patients (26.6%), resulting in a considerable number of pathological stage I patients. Tumor stage and basic characteristics are listed in Table 1.
Postoperative outcomes
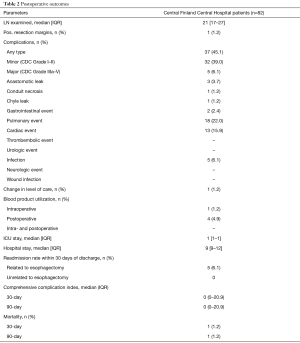
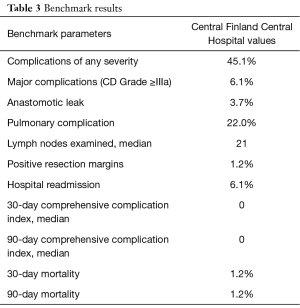
Any morbidity at 30 days after surgery was observed in 37 (45.1%) patients, with 5 patients (6.1%) experiencing major complications (Clavien-Dindo ≥3a) (Table 2). A fully anticoagulated patient with cardiomyopathy faced sudden death in the early morning of the planned discharge day (postoperative day 9) due to sudden major intra-abdominal bleeding. The cause of death was confirmed by autopsy. No additional deaths were observed and, therefore, both the 30- and 90-day mortality was 1.2%. The most common postoperative complications were pulmonary (n=18, 22.0%), of which 9 (11.0%) were pneumonias. The second most common complication was cardiac events (n=13, 15.9%), mainly atrial fibrillations (n=12) requiring medical treatment. Anastomosis leak was observed in 3 patients (3.7%); 2 type 1 leaks were treated with antibiotics and 1 type 2 leak with esophageal stent in a patient with initial symptoms after discharge and who was readmitted and later required treatment in the ICU. Focal type 1 necrosis of the gastric conduit was observed in one patient treated with antibiotics. Other complications were rather uncommon (Table 2).
Full table
Median ICU and hospital stay were 1 (IQR, 1–1) and 9 (IQR, 9–12) days, respectively. Five patients (6.1%) were readmitted to hospital within 30 days of discharge. Readmission was related to dysphagia (1 patient), delayed gastric emptying (3 patients), and anastomosis leak (1 patient). All these patients were treated conservatively or endoscopically. An additional four patients (4.9%) were readmitted between 30 and 90 days of discharge for reasons related to formation of anastomosis stricture in two patients (treated with endoscopic dilatation), upper gastric pain in one and dysphagia in one patient (resolved without treatment). One patient with a complete response to neoadjuvant therapy in the primary tumor, but who had atypical cells in the resection margin, presented with local recurrence 10 months after the primary operation without distant metastases, and was treated with re-resection and colon interposition. Blood transfusions were administered intraoperatively in 1 patient (1.2%) and postoperatively in 4 patients (4.9%) (Table 2).
Benchmark values
The suggested benchmark values are compared with the results from our center in Tables 3,4. At our center, older patients with more comorbidity had lower rates of all complications (45.1% vs. 56.0%) and severe complications (6.1% vs. 26.9%). Of the listed benchmark parameters, only lymph node yield was below the benchmark value at our center (median 21 vs. benchmark ≥23).
Full table
Survival
Overall survival in our series versus the benchmark patients was 88.3% vs. 85.5% at 1 year, and 67.7% vs. 62.2% at 3 years.
Discussion
The present study revealed superior outcomes after MIE at a medium volume center compared to the recently reported “best achievable” results at high-volume centers. We suggest that the benchmark values that have been set should be viewed critically, with a shift in morbidity and 90-day mortality.
The most important difference between our and the benchmark study was seen in the rate of anastomotic leak. The reported leak rate of 15.9% in the benchmark study was highly associated with fatal outcomes and major complications (1). The benchmark cut-off was set at 20.0%. Both of these rates are significantly higher than previously reported rates of around 10% (10,15,18,25,26). One potential explanation for this is the high number of cervical anastomoses in the benchmark study (43.7% vs. 13.4% in our report), even though the rate of distal tumors was nearly identical. Previous studies have shown higher postoperative mortality associated with neck anastomosis (25,27). Also, part of the benchmark centers, as addressed by the authors, were still in the middle of their learning curve, and also changing the approach used during the study period (28). The results can be further improved with omental reinforcement flaps, also used routinely at our center, which were shown to decrease overall leak rates (29). The best example of refinements to the MIE procedure was described by the pioneer of this technique, James Luketich who, during a 15-year span, adjusted the width of the gastric conduit, included the omental flap, and converted from neck anastomosis to intrathoracic anastomosis, improving the results (18). Following these principles, the leak rate at a medium volume center is now less than 5%. Until refinements are made and the MIE technique is mastered, it is too early to set benchmark values for MIE.
The second important point was the mortality in the benchmark study, with an overall 30-day mortality of 2.1% and 5.2% at 90 days, and 0.9% and 2.4% among the benchmark low-risk patients, respectively (1). The benchmark values were set at 1.0% and 4.6% at 30 and 90 days (1). The 90-day mortality rate of 4.1% after MIE in Finland and Sweden was recently revealed at the population level (12). Accordingly, the “best possible” 90-day mortality was higher than the mortality in the Scandinavian population-based study. Previously, the top 10% of hospitals in the US reported a 90-day mortality rate of 2.2% (5). A 90-day mortality rate of 1%, as in our study, has been reported as well (30). The benchmark value, after the learning curve, should therefore be no more than 2–3%.
The most common group of complications after esophagectomy is pulmonary, and the incidence has been successfully reduced by MIE (11). One possible explanation for the reduced number pulmonary complications in our series was the rate of neck anastomosis, for which a constantly higher rate of recurrent laryngeal nerve trauma has been reported (31). In the open era, this injury was related to the increased incidence of pulmonary complications, length of ventilator time, and ICU stay (32). The second reason, despite having older and comorbid patients, could be an enhanced mobilization program (33). This program, together with lower rate of complications compared to the benchmark series, has reduced the median ICU stay by 1 day and median hospital stay by 4 days. Overall, we should set the benchmark values for ICU and hospital stays as well, aiming for 1 and 9 days, respectively.
The number of lymph nodes examined, with a median of 21, was the only benchmark parameter in our series that did not reach the set cut-off value of ≥23. However, the pathological stage distributions between our series and those of benchmark patients were very similar, with equivalent 1- and 3-year survival rates, reflecting a technically good oncological surgery.
The inclusion criterion for the participating hospitals was a caseload of more than 20 esophagectomies annually (1). Hospital volume (>20 cases/year) has been shown to reduce the operative mortality (34). This mortality seems, however, to be more dependent on surgeon volume (35). Both annual and overall case load have had an effect on the outcomes, with the best results reached after an annual volume of 20 cases and an overall volume between 119 and 200 cases (36-38). These numbers, at our center, were reached by the operating surgeon (ES) performing a mean number of 16 MIEs annually after a learning curve at a high-volume center with total of more than 200 open and 50 minimally invasive esophagectomies (19,20). In the benchmark study, the calculated annual number of MIEs per center was 16. Most likely, these operations were performed with at least 2 surgeons per center, reducing the mean annual caseload to below 10. With a procedure as complex as MIE, especially during the learning curve phase, this is not the caseload needed to reach the “best achievable results”.
The major weakness in this study is that this is a relatively small single center study. On the other hand, this study is a snapshot of all esophageal cancer operations performed at a medium-volume center after a learning curve. Inclusion of all cancer patients regardless of age or comorbidities and including those who received hybrid procedures and conversions, addresses the actual life situation. In all cases during the study period, a MIE or hybrid procedure was an intention-to-treat operation, making selection bias unlikely. Furthermore, this data was collected prospectively and reconfirmed extremely carefully by a third researcher who was not responsible for treating these patients. In the data collection for complications, we strictly followed suggestions by ECCG as did Schmidt et al. Complete early follow-up 4 weeks after discharge at our center excludes the chance for missing any significant complications. Nationwide compulsory databases enabled us to receive complete long-term mortality data.
According to our results and those from previous (39) studies, the suggested benchmark values published by Schmidt et al. do not represent the “best achievable outcome” for MIE. This is highlighted by the benchmark study itself with the presented variations in the rate of overall or severe complications between those 13 participating centers. These benchmark levels for MIE should be critically evaluated in future studies at high-volume centers with completed learning curves.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Central Finland Hospital district. Because of the retrospective nature of the study, patient informed consent or ethical statement was not required.
References
- Schmidt HM, Gisbertz SS, Moons J, et al. Defining Benchmarks for Transthoracic Esophagectomy: A Multicenter Analysis of Total Minimally Invasive Esophagectomy in Low Risk Patients. Ann Surg 2017;266:814-21. [Crossref] [PubMed]
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524-48. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v50-7. [Crossref] [PubMed]
- In H, Palis BE, Merkow RP, et al. Doubling of 30-Day Mortality by 90 Days After Esophagectomy: A Critical Measure of Outcomes for Quality Improvement. Ann Surg 2016;263:286-91. [Crossref] [PubMed]
- Talsma AK, Lingsma HF, Steyerberg EW, et al. The 30-day versus in-hospital and 90-day mortality after esophagectomy as indicators for quality of care. Ann Surg 2014;260:267-73. [Crossref] [PubMed]
- Hagen JA, DeMeester SR, Peters JH, et al. Curative resection for esophageal adenocarcinoma: analysis of 100 en bloc esophagectomies. Ann Surg 2001;234:520-30; discussion 530-1. [Crossref] [PubMed]
- Pennathur A, Luketich JD. Minimally invasive esophagectomy: short-term outcomes appear comparable to open esophagectomy. Ann Surg 2012;255:206-7. [Crossref] [PubMed]
- Sihag S, Wright CD, Wain JC, et al. Comparison of perioperative outcomes following open versus minimally invasive Ivor Lewis oesophagectomy at a single, high-volume centre. Eur J Cardiothorac Surg 2012;42:430-7. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Kauppila JH, Helminen O, Kyto V, et al. Short-Term Outcomes Following Minimally Invasive and Open Esophagectomy: A Population-Based Study from Finland and Sweden. Ann Surg Oncol 2018;25:326-32. [Crossref] [PubMed]
- Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus 2009;22:1-8. [Crossref] [PubMed]
- Rössler F, Sapisochin G, Song G, et al. Defining Benchmarks for Major Liver Surgery: A multicenter Analysis of 5202 Living Liver Donors. Ann Surg 2016;264:492-500. [Crossref] [PubMed]
- Kauppi J, Rasanen J, Sihvo E, et al. Open versus minimally invasive esophagectomy: clinical outcomes for locally advanced esophageal adenocarcinoma. Surg Endosc 2015;29:2614-9. [Crossref] [PubMed]
- Brunelli A, Pompili C, Berardi R, et al. Performance at preoperative stair-climbing test is associated with prognosis after pulmonary resection in stage I non-small cell lung cancer. Ann Thorac Surg 2012;93:1796-800. [Crossref] [PubMed]
- Helminen O, Mrena J, Sihvo E. Near-infrared image-guided lymphatic mapping in minimally invasive oesophagectomy of distal oesophageal cancer. Eur J Cardiothorac Surg 2017;52:952-7. [Crossref] [PubMed]
- Zhang J, Wang R, Liu S, et al. Refinement of minimally invasive esophagectomy techniques after 15 years of experience. J Gastrointest Surg 2012;16:1768-74. [Crossref] [PubMed]
- Sihvo EI, Rasanen JV, Knuuti MJ, et al. Adenocarcinoma of the esophagus and the esophagogastric junction: positron emission tomography improves staging and prediction of survival in distant but not in locoregional disease. J Gastrointest Surg 2004;8:988-96. [Crossref] [PubMed]
- Salo J, Sihvo E, Kauppi J, et al. Boerhaave's syndrome: lessons learned from 83 cases over three decades. Scand J Surg 2013;102:271-3. [Crossref] [PubMed]
- Mrena J, Mattila A, Böhm J, et al. Surgical care quality and oncologic outcome after D2 gastrectomy for gastric cancer. World J Gastroenterol 2015;21:13294-301. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Slankamenac K, Graf R, Barkun J, et al. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013;258:1-7. [Crossref] [PubMed]
- Kassis ES, Kosinski AS, Ross P Jr, et al. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg 2013;96:1919-26. [Crossref] [PubMed]
- Jones CE, Watson TJ. Anastomotic Leakage Following Esophagectomy. Thorac Surg Clin 2015;25:449-59. [Crossref] [PubMed]
- Degisors S, Pasquer A, Renaud F, et al. Are Thoracotomy and/or Intrathoracic Anastomosis Still Predictors of Postoperative Mortality After Esophageal Cancer Surgery?: A Nationwide Study. Ann Surg 2017;266:854-862. [Crossref] [PubMed]
- Nilsson M, Kamiya S, Lindblad M, et al. Implementation of minimally invasive esophagectomy in a tertiary referral center for esophageal cancer. J Thorac Dis 2017;9:S817-25. [Crossref] [PubMed]
- Sepesi B, Swisher SG, Walsh GL, et al. Omental reinforcement of the thoracic esophagogastric anastomosis: an analysis of leak and reintervention rates in patients undergoing planned and salvage esophagectomy. J Thorac Cardiovasc Surg 2012;144:1146-50. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP, Grosser R, et al. Attaining Proficiency in Robotic-Assisted Minimally Invasive Esophagectomy While Maximizing Safety During Procedure Development. Innovations (Phila) 2016;11:268-73. [Crossref] [PubMed]
- van Workum F, Berkelmans GH, Klarenbeek BR, et al. McKeown or Ivor Lewis totally minimally invasive esophagectomy for cancer of the esophagus and gastroesophageal junction: systematic review and meta-analysis. J Thorac Dis 2017;9:S826-33. [Crossref] [PubMed]
- Scholtemeijer MG, Seesing MF, Brenkman HJ, et al. Recurrent laryngeal nerve injury after esophagectomy for esophageal cancer: incidence, management, and impact on short- and long-term outcomes. J Thorac Dis 2017;9:S868-78. [Crossref] [PubMed]
- Markar SR, Naik R, Malietzis G, et al. Component analysis of enhanced recovery pathways for esophagectomy. Dis Esophagus 2017;30:1-10. [Crossref] [PubMed]
- Fuchs HF, Harnsberger CR, Broderick RC, et al. Mortality after esophagectomy is heavily impacted by center volume: retrospective analysis of the Nationwide Inpatient Sample. Surg Endosc 2017;31:2491-7. [Crossref] [PubMed]
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. The New England journal of medicine 2003;349:2117-2127. [Crossref] [PubMed]
- Markar SR, Mackenzie H, Lagergren P, et al. Surgical Proficiency Gain and Survival After Esophagectomy for Cancer. J Clin Oncol 2016;34:1528-36. [Crossref] [PubMed]
- Mamidanna R, Ni Z, Anderson O, et al. Surgeon Volume and Cancer Esophagectomy, Gastrectomy, and Pancreatectomy: A Population-based Study in England. Ann Surg 2016;263:727-32. [Crossref] [PubMed]
- van Workum F, Stenstra MH, Berkelmans GH, et al. Learning Curve and Associated Morbidity of Minimally Invasive Esophagectomy: A Retrospective Multicenter Study. Ann Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Dunst CM, Swanstrom LL. Minimally invasive esophagectomy. J Gastrointest Surg 2010;14 Suppl 1:S108-14. [Crossref] [PubMed]