Clinicopathological characteristics and prognosis of pulmonary pleomorphic carcinoma: a population-based retrospective study using SEER data
Introduction
Pulmonary pleomorphic carcinoma (PPC) is a rare, poorly differentiated, epithelial cell tumor comprising 0.1–0.6% of all lung malignancies (1). PPC is classified by the World Health Organization as a lung tumor including at least 10% giant cell and/or spindle cell elements plus components of adenocarcinoma, squamous cell carcinoma, and large cell carcinoma (2,3). PPC has a more aggressive clinical course than other subtypes of NSCLC (4,5). Because it is rare, few studies of PPC patients are available, and most are reports of surgical treatment. Data on the clinicopathological characteristics and prognosis of PPC are limited. Mochizuki et al. (4) reviewed 70 cases of surgically resected PPC, describing it as an aggressive disease. A retrospective study of 33 PPC patients by Nishida et al. found that prognosis following surgical resection was worse than that of other NSCLC patients (6). This study reviewed the clinical and pathological characteristics and survival of PCC patients in a large national population-based database to better understand its clinical behavior and factors that affect survival.
Methods
Data extraction
Data on PPC patients diagnosed and treated between 2004 and 2014 were extracted from the SEER database (http://seer.cancer.gov/) database using SEER*Stat software version 8.3.5 (https://seer.cancer.gov/seerstat/). The study cohort included patients with the International Classification of Diseases for Oncology, third edition (ICD-O-3) histology code 8022/3. Eligible patients had a single primary tumor, sequence number 0 or 1; histologically confirmed malignant PPC, complete follow-up data, and known age and race. Signed authorization and permission were obtained from SEER to access and use the dataset. Approval was waived by the local ethics committee, as SEER data is publicly available and de-identified.
Variables
The SEER data included patient age, sex, race, tumor grade, primary site, year of diagnosis, tumor size, TNM stage of the primary tumor, historic stage A, surgical resection (yes or no), type of lung surgery (e.g., pneumonectomy, lobectomy, sublobar resection), radiation (yes or no), chemotherapy (yes or no), survival data, and vital status. The TNM stage was manually adjusted following the AJCC eighth edition criteria (7). Patient age at diagnosis was converted to 10-year categorical intervals when constructing predictive nomograms. Types of lung surgery included an “other” category.
Survival data
Overall survival (OS) was the interval from the date of diagnosis until the date of death from any cause or the date of the last follow-up. Patients who survived less than 1 month were coded in the SEER database with a survival time of zero. A survival of 0.5 months was assigned to those patients following standard epidemiological convention.
Statistical analysis
Cumulative survival curves for each patient variable were constructed using the Kaplan-Meier method and were compared using the log-rank test. Patient variables with prognostic value were identified by Cox proportional hazards regression and reported as hazard ratios (HRs). Variables with P values <0.1 in univariate analysis (UVA) were included in multivariate analysis (MVA). Nomograms were derived from the results of MVA as previously reported (8). Prediction error was estimated with 200 bootstrap samples. The concordance index (c-index) and calibration plots were used to evaluate model performance. The c-index measures discrimination and from 0.5 to 1.0. A larger c-index indicates better accuracy to distinguish subject outcomes (9,10). Nomogram plot calibration was used to estimate the overall agreement between predicted and the observed survival as previously described (11). Statistical analysis was performed using R version 3.4.3 software (http://www.r-project.org/). The R package included survival, rms and ggplot2. Statistical significance was set at a two-sided P value <0.05.
Results
Patient demographic, clinical, and pathological characteristics
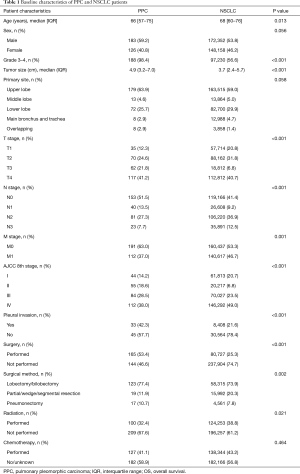
A total of 320,510 eligible NSCLC patients were identified in the SEER database, including 309 (0.10%) with PPC. As shown in Table 1, the PPC patients included 183 men and 126 women with a median age of 66 and interquartile range (IQR) of 57–75 years. The majority, 245 or 79.3%, were White. The most common PPC site was the upper lobe, in 179 patients (63.9%) followed by the lower lobe in 72 (25.7%), and the middle lobe in 13 (4.6%). The histologic grades included 1.6% grades I and II (well or moderately differentiated), 77.0% grade III (poorly differentiated), and 21.5% grade IV (undifferentiated). Most patients (38.0%) were AJCC stage IV, 28.5% were stage III, 14.2% were stage I, and 18.6% were stage II. PPC patients had significantly larger and less well differentiated tumors, more extensive pleural invasion, a higher percentage of radical surgical resection, less N+ disease, and fewer distant metastases than NSCLC patients (P<0.001).
Full table
Survival
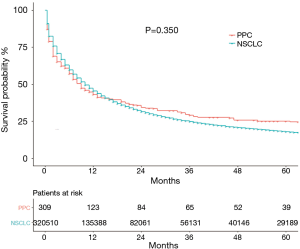
The median OS of the 309 PCC patients with PPC in the current study was 9 (95% CI: 6.69–11.31) months, and 5-year OS was 25.1% (95% CI: 23.6–26.6%). In the whole NSCLC cohort, median OS was 10 (95% CI: 9.92–10.08) months with a 5-year OS of 18.0% (95% CI: 17.8–18.2%). The Kaplan-Meier curves of OS are shown in Figure 1. A total of 221 patients died during the follow-up period, 185 deaths were cancer-specific, attributable to PPC. Thirty-six were from other causes, including COPD and allied conditions (27.8%) and heart disease (13.9%).
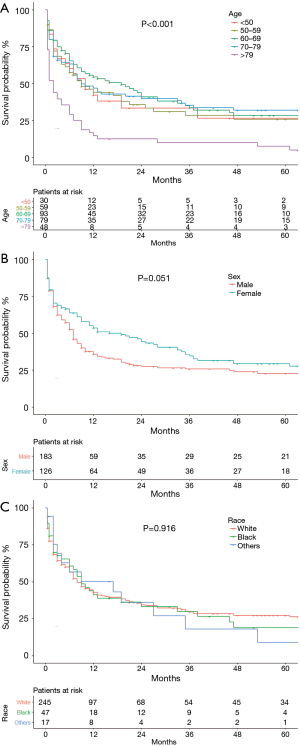
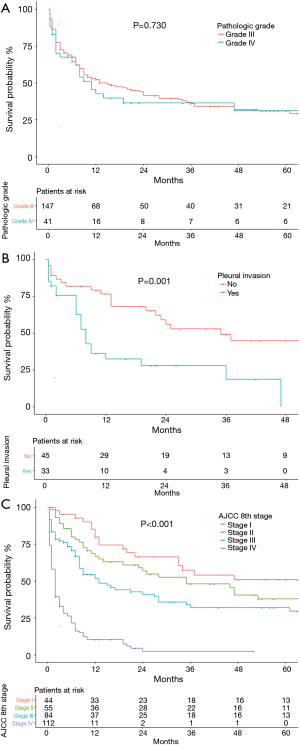
Cumulative survival of patients of different age groups, sex, and race are shown in Figure 2. The survival probability of patients <50, 50–59 and 70–79 group years of age did not differ significantly (Figure 2A). OS was significantly worse in patients ≥80 years of age than in the other age groups (P=0.004), and the OS of patients 60–69 years of age was high relative to younger patients. Women (Figure 2B) tended to have a better OS than men (P=0.051). The OS of the racial groups did not differ (P=0.916, Figure 2C). Patients with grade I or II well or moderately differentiated tumors were not included in the survival analysis because only three cases were identified. OS did not differ in patients with grade III (poorly differentiated) and grade IV (undifferentiated) tumors (P=0.730, Figure 3A). Data on pleural invasion became available in 2010, and was found in 33 patients (42.3%), including some with early stage tumors. Patients with tumors extending to the visceral or parietal pleural surface had significantly worse OS than those without invasion (P=0.001, Figure 3B). OS varied significantly with AJCC stage (P<0.001, Figure 3C). Median survival was 86 (95% CI: 12.14–159.86) months and 5-year OS 50.9% in stage I patients. The corresponding values were 35 (95% CI: 15.46–54.54) months and 37.8% for stage II, 13 (95% CI: 5.96–20.04) months and 32% for stage III, and 2 (95% CI: 1.52–2.48) months and 2.2% for stage IV patients.
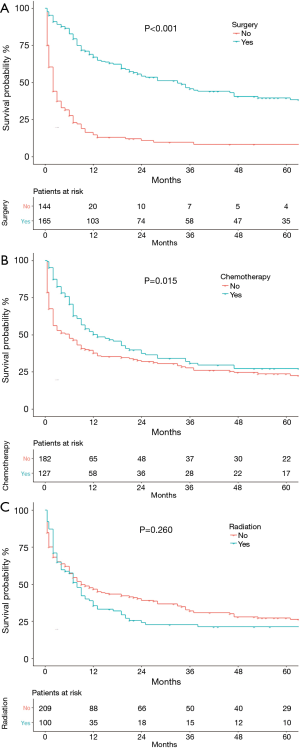
As shown in Figure 4, patients who underwent surgery (P<0.001, Figure 4A) or chemotherapy (P=0.015, Figure 4B) had significantly better OS than those who did not. Receiving radiation therapy did not significantly affect OS (P=0.260, Figure 4C). OS did differ with surgical procedure (P=0.012). The median OS for patients with lobectomies was 46 (95% CI: 30.15–61.85) months vs. 20 (95% CI: 1.37–38.63) months for partial/wedge/segmental resection vs. 12 (95% CI: 3.93–20.07) months for pneumonectomy, (P=0.027). Nodal status was pN0 in 105 (64.4%) patients and pN+ in 58 (35.6%). Median OS was 37 (95% CI: 18.03–55.97) months in the pN0 patients and 28 (95% CI: 11.21–44.79) months in pN+ patients (P=0.766).
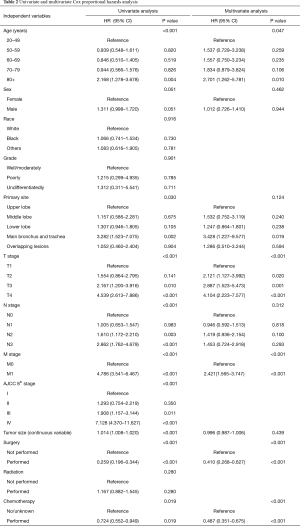
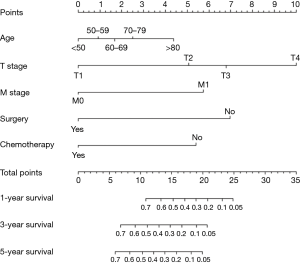
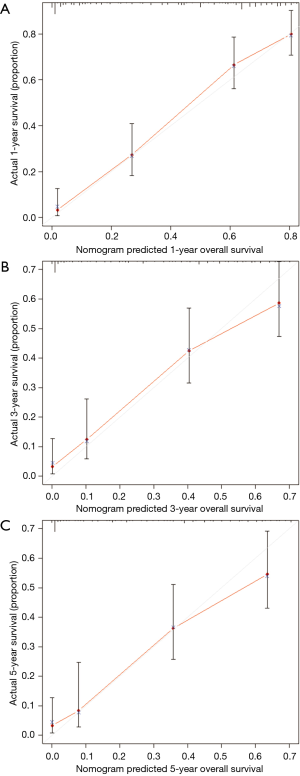
MVA included all covariates with a P value <0.1 in UVA. As pleural invasion and AJCC stage were correlated with TNM stage, they were not included in the MVA to avoid collinearity among factors. Results of the UVA and MVA Cox proportional hazards regression are shown in Table 2. MVA found that age (P=0.047), T stage (P<0.001), M stage (P<0.001), surgery (P<0.001), and chemotherapy (P<0.001) were independently able to predict survival. A nomogram including the variables independently related to survival is shown in Figure 5. One-, 3-, or 5-year OS can be estimated by adding the points corresponding to the patient’s characteristics. The nomogram had a c-index of 0.798, which indicates relatively good discriminative ability. Calibration plots of the nomogram prediction accuracy are shown in Figure 6, and show good agreement of actual and predicted 1-, 3-, and 5-year OS, with a slope close to 45.
Full table
Discussion
Because of its rarity, few data on the clinical features and prognosis of PPC are available. A large cohort of 309 PPC patients included in the SEER database was analyzed to study this cancer. The aim was to describe the clinicopathological characteristics of PPC patients and compare them with other NSCLC populations. Age, T stage, M stage, surgery, and chemotherapy were found to be independently associated with survival, and a nomogram derived from the Cox regression model was built to accurately predict the prognosis of PPC patients in the study cohort.
PPC comprised 0.1% of the NSCLC patients retrieved from the SEER database, which is consistent with the 0.1–0.3% previously reported by Chang et al. (1). In the SEER population, the median age at diagnosis was 66 years, and despite racial and regional differences in other reports, is consistent with the median 60–70 years of age in previous studies. Men made up 59.2% of the SEER cohort, and ranged from 67% to 95% in other studies, indicating that PPC has a strong male predominance (1,4-6,12).
A group of clinical characteristics unique to PPC and reported in this and in other studies includes occurrence in white patients, originating in the upper lobe, poor differentiation, and AJCC stage IV (5,13-15). In line with previous reports, PPCs frequently invaded the pleura or chest wall, which made resection difficult (5). PPC patients also had significantly larger and less well differentiated tumors, more frequent treatment by radical surgical resection, less N+ disease, and fewer distant metastases compared with other NSCLC patients (P<0.001). Pelosi et al. (15) reported PPCs with tumor diameters >3 cm in 84% of their patients, and rarely encountered small tumors with diameters <3 cm. In the SEER sample, the median PCC tumor diameter was 5.0 cm, comparing with 3.8 cm in other NSCLCs (P<0.001). The size difference may result from a lack of early PPC-specific manifestations combined with rapid growth. Previous estimates of PCC growth by computed tomography CT using a method described by Schwartz found tumor doubling times of <30 days, which is shorter than those of the common types of NSCLC (13,16-18).
The patient variables that influence prognosis and long-term survival of PPC are controversial. PPC may have a more aggressive clinical course and poorer outcome than other NSCLCs (4,19,20), but Yamamoto et al. found no difference in the OS of PPC and other NSCLC patients (21). The median OS of the PPC patients in this study was 9 vs. 10 months in those with NSCLC. In this analysis, which was not case-matched, the difference in OS was not significant. PPCs did have a poor prognosis even when early stage disease was treated effectively. The median OS for advanced-stage PPCs was extremely poor, 13 months for stage III and 2 months for stage IV, which resulted in a sharp decrease in survival probability in the first year after diagnosis. Distant metastasis involved a larger population in other NSCLC than in PPC, which did narrow the difference in median OS.
Age, T stage, M stage, tumor size, AJCC stage and surgery, remained significantly associated with survival following MVA, and the Cox regression results were used to construct a nomogram to predict 1-, 3-, and 5-year OS. The nomogram is easy to use and would be a useful clinical tool for physicians and patients, as it showed that surgery had a strong impact on OS. As in other NSCLCs, surgery is the preferred treatment for stage I, II, and some stage III PPCs. Adequate follow-up therapy after surgery can improve prognosis (17), and the benefits of surgery are greater with resection at an early stage (4). The surgical procedures include lobectomy, pneumonectomy, and partial, wedge, or segmental resection. Lobectomy is widely accepted for complete resection because of its pulmonary preservation. The benefits of surgery are less clear in patients with nodal metastasis. Raveglia et al. (19) and Yuki et al. (20) reported statistically significant differences in the OS of patients with pN0 and pN+ tumors, but Fujisaki et al. found that the presence of lymph node metastasis at surgery did not predict OS or disease-free survival in another series of 44 patients (22). A 9-month benefit in median OS was seen in pN0 patients compared with pN1 patients who underwent surgery, but the difference was not statistically significant.
Previous studies reported that postoperative adjuvant therapy contributed little to improving the prognosis of PPC patients (5,23). Radiotherapy did not influence the OS of the patients in this study, but chemotherapy was independently associated with OS in both univariate and multivariate Cox proportional hazards analysis. Bae et al. (23) reported a poor response and high recurrence in advanced PPC patients treated with chemotherapy regimens effective against NSCLC. That study included 13 patients treated between August 1999 and November 2006. Since then, new chemotherapeutic agents have been approved and supportive treatment developed to reduce the effects of adverse reactions has improved treatment response. Tamura et al. reported relatively long-term survival in two PPC patients treated with a carboplatin, pemetrexed, bevacizumab regimen (24), and Lee et al. reported strong responses to treatment with mesna, doxorubicin, ifosfamide, and dacarbazine (MAID) in two platinum-refractory PPC patients (25).
Novel targeted treatments are under investigation. In an evaluation of 42 pleomorphic carcinomas, Chang et al. (26) identified EGFR somatic mutations in 23.8% of the tumor tissues and p53 in 28.5%. In a similar study, Jia et al. (27) found EGFR mutations in 15.7% and KRAS mutations in 14.3% of a series of Chinese PPC patients. EGFR tyrosine kinase inhibitors may thus be an alternative treatment, improving the prognosis of PPCs with EGFR mutations (28).
The study limitations include the lack of smoking history, CT findings, and data on recurrence and presence of genetic mutations in the SEER records. Those variables could not be evaluated in our study. Other studies have reported that a history of heavy tobacco use increased susceptibility to PPC (4) and that the presence of a large central area or cavity with low-attenuation (>25% of the tumor) on CT predicted poorer disease-free survival (4). The treatment variables that influenced prognosis could not be fully evaluated because complete information on surgical procedures, chemotherapy regimens, and radiation dose/technology were not included in the SEER records. The performance status of patients, which is the valid factor influencing the prognosis of patients with lung cancer, was also not recorded in the SEER database and therefore was not included in our analyses (29). Finally, the retrospective study design and use of nonrandomized data may have introduced confounding in analysis of covariate effects. Large prospective multicenter studies could provide a more comprehensive analysis of risk factors and suggest novel treatment rationales for PPC patients. To our knowledge this study is currently the largest population-based PPC series and provides a novel, predictive model of PPC prognosis that performed well in this case series.
Conclusions
PPC presented with distinct clinicopathological characteristics compared with other NSCLC, including advanced age, male dominance, poor differentiation and large tumor size. Age, T stage, M stage, surgery, and chemotherapy were independently associated with OS. A nomogram predicted 1-, 3- and 5-year OS of these patients.
Acknowledgments
Funding: This work was supported by the Research Program of Shanghai Health and Family Planning Commission (201640102) (www.wsjsw.gov.cn), the Suzhou Industry Technology Innovation Program (SYSD2017172) (www.szkj.gov.cn), and the Shanghai Sailing Program (No. 17YF1402400) (www.stcsm.gov.cn).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Approval was waived by the local ethics committee, as SEER data is publicly available and de-identified.
References
- Chang YL, Lee YC, Shih JY, et al. Pulmonary pleomorphic (spindle) cell carcinoma: peculiar clinicopathologic manifestations different from ordinary non-small cell carcinoma. Lung Cancer 2001;34:91-7. [Crossref] [PubMed]
- Brambilla E, Travis WD, Colby TV, et al. The new World Health Organization classification of lung tumours. Eur Respir J 2001;18:1059-68. [Crossref] [PubMed]
- Travis WD. Sarcomatoid neoplasms of the lung and pleura. Arch Pathol Lab Med 2010;134:1645-58. [PubMed]
- Mochizuki T, Ishii G, Nagai K, et al. Pleomorphic carcinoma of the lung: clinicopathologic characteristics of 70 cases. Am J Surg Pathol 2008;32:1727-35. [Crossref] [PubMed]
- Chen F, Sonobe M, Sato T, et al. Clinicopathological characteristics of surgically resected pulmonary pleomorphic carcinoma. Eur J Cardiothorac Surg 2012;41:1037-42. [Crossref] [PubMed]
- Nishida A, Abiru H, Hayashi H, et al. Clinicoradiological outcomes of 33 cases of surgically resected pulmonary pleomorphic carcinoma: correlation with prognostic indicators. Eur Radiol 2016;26:25-31. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Sun F, Ma K, Yang X, et al. A nomogram to predict prognosis after surgery in early stage non-small cell lung cancer in elderly patients. Int J Surg 2017;42:11-6. [Crossref] [PubMed]
- Koepsell TD, Weiss NS. Epidemiologic Methods: Studying the Occurrence of Illness. UK: Oxford University Press, 2014.
- Ma K, Sun F, Yang X, et al. Prognosis of patients with primary malignant main stem bronchial tumors: 7,418 cases based on the SEER database. Onco Targets Ther 2017;11:83-95. [Crossref] [PubMed]
- Wang S, Ma K, Wang Q, et al. The revised staging system for malignant pleural mesothelioma based on surveillance, epidemiology, and end results database. Int J Surg 2017;48:92-8. [Crossref] [PubMed]
- Miyahara S, Hamasaki M, Hamatake D, et al. Clinicopathological analysis of pleomorphic carcinoma of the lung: diffuse ZEB1 expression predicts poor survival. Lung Cancer 2015;87:39-44. [Crossref] [PubMed]
- Fishback NF, Travis WD, Moran CA, et al. Pleomorphic (spindle/giant cell) carcinoma of the lung. A clinicopathologic correlation of 78 cases. Cancer 1994;73:2936-45. [Crossref] [PubMed]
- Rossi G, Cavazza A, Sturm N, et al. Pulmonary carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements: a clinicopathologic and immunohistochemical study of 75 cases. Am J Surg Pathol 2003;27:311-24. [Crossref] [PubMed]
- Pelosi G, Sonzogni A, De Pas T, et al. Review article: pulmonary sarcomatoid carcinomas: a practical overview. Int J Surg Pathol 2010;18:103-20. [Crossref] [PubMed]
- Detterbeck FC, Gibson CJ. Turning gray: the natural history of lung cancer over time. J Thorac Oncol 2008;3:781-92. [Crossref] [PubMed]
- Ito K, Oizumi S, Fukumoto S, et al. Clinical characteristics of pleomorphic carcinoma of the lung. Lung Cancer 2010;68:204-10. [Crossref] [PubMed]
- Schwartz M. A biomathematical approach to clinical tumor growth. Cancer 1961;14:1272-94. [Crossref] [PubMed]
- Raveglia F, Mezzetti M, Panigalli T, et al. Personal experience in surgical management of pulmonary pleomorphic carcinoma. Ann Thorac Surg 2004;78:1742-7. [Crossref] [PubMed]
- Yuki T, Sakuma T, Ohbayashi C, et al. Pleomorphic carcinoma of the lung: a surgical outcome. J Thorac Cardiovasc Surg 2007;134:399-404. [Crossref] [PubMed]
- Yamamoto S, Hamatake D, Ueno T, et al. Clinicopathological investigation of pulmonary pleomorphic carcinoma. Eur J Cardiothorac Surg 2007;32:873-6. [Crossref] [PubMed]
- Fujisaki A, Aoki T, Kasai T, et al. Pleomorphic Carcinoma of the Lung: Relationship Between CT Findings and Prognosis. AJR Am J Roentgenol 2016;207:289-94. [Crossref] [PubMed]
- Bae HM, Min HS, Lee SH, et al. Palliative chemotherapy for pulmonary pleomorphic carcinoma. Lung Cancer 2007;58:112-5. [Crossref] [PubMed]
- Tamura T, Ohara G, Kagohashi K, et al. Pemetrexed and bevacizumab-containing chemotherapy for pleomorphic carcinoma of the lung. Mol Clin Oncol 2016;4:616-8. [Crossref] [PubMed]
- Lee KW, Kim YJ, Kim JH, et al. Two consecutive cases of platinum-refractory pulmonary pleomorphic carcinoma that showed dramatic responses to MAID (mesna, doxorubicin, ifosfamide and dacarbazine) chemotherapy. Jpn J Clin Oncol 2011;41:430-3. [Crossref] [PubMed]
- Chang YL, Wu CT, Shih JY, et al. EGFR and p53 status of pulmonary pleomorphic carcinoma: implications for EGFR tyrosine kinase inhibitors therapy of an aggressive lung malignancy. Ann Surg Oncol 2011;18:2952-60. [Crossref] [PubMed]
- Jia X, Chen G. EGFR and KRAS mutations in pulmonary pleomorphic carcinoma and their correlation with clinicopathologic features. Contemp Oncol (Pozn) 2015;19:22-7. [Crossref] [PubMed]
- Ushiki A, Koizumi T, Kobayashi N, et al. Genetic heterogeneity of EGFR mutation in pleomorphic carcinoma of the lung: response to gefitinib and clinical outcome. Jpn J Clin Oncol 2009;39:267-70. [Crossref] [PubMed]
- Gajra A, Marr AS, Ganti AK. Management of patients with lung cancer and poor performance status. Journal of the National Comprehensive Cancer Network 2014;12:1015-25. [Crossref] [PubMed]