Clinicopathological features of lung cancer in patients with rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory disease that predominately affects articular and related structures. The prevalence of RA in the general population ranges from 0.5% to 2% (1). Approximately 40% of patients with RA have some types of extra-articular manifestations, including myocarditis, pneumonitis, serositis, valvulitis, myositis, arteritis, peripheral neuritis, and hematological changes (2). The lung is a frequent site of extra-articular involvement. RA could affect the pulmonary parenchyma, airways, pleura, and vasculature. A growing body of evidence has suggested an elevated risk of lung cancer (LC) in the RA population (3-5). However, existing literature regarding RA associated LC have been hampered by the limited sample size, lack of a control group of general LC patients, and absence of information about genetic mutation status and anti-cancer treatment outcomes. We therefore performed a retrospective cohort study to identify the potential clinicopathologic differences of LC in RA patients and in patients without systemic connective tissue disorder (CTD). Furthermore, as smoking is a shared risk for both LC and RA, we compared the clinicopathologic characteristics of LC patients with RA according to their smoking status, aiming to explore the role of smoking in RA associated LC.
Methods
Study population and data collection
A total of 65 LC patients with RA and 13,533 LC patients without comorbid systemic CTD from 2000 to 2016 were admitted to Peking Union Medical College Hospital, Chinese Academy of Medical Sciences. After excluding patients who were lost to follow-up and whose pathological or radiological data could not be retrieved, 44 LC patients with RA were identified. In consideration of the increased incidence of and changed clinicopathologic characteristics of LC over the past 20 years, four controls were randomly selected from the group of LC patients without autoimmune CTD and matched to one case in the group of LC patients with RA by diagnosis year. Finally, 44 LC patients with RA and 176 LC patients without CTD were included in this study.
Clinicopathologic characteristics of the patients were reviewed, including gender, age at the time of diagnosis of RA and LC, smoking history, performance status, disease stage, tumor histology, grade and genetic mutation status, tumor location, high-resolution computed tomography (HRCT) findings, serologic testing for rheumatoid factor (RF), anti-cyclic citrullinated peptides (CCP) antibodies, and antinuclear antibodies (ANA), and treatments for RA and LC.
The diagnosis of LC was confirmed by cytological or histological examinations. The mutational analysis was performed on the DNA samples extracted from surgically resected, biopsied, or cytologic specimens of lung adenocarcinomas. Epidermal growth factor receptor (EGFR) mutation status was tested by qualitative real-time polymerase chain reaction (PCR) using EGFR RGQ PCR Kit. ALK fusion was tested by immunohistochemistry using VENTANA ALK (D5F3) CDx Assay. The clinical stage was determined based on the international TNM criteria for cancer staging. Performance status was assessed according to the Eastern Cooperative Oncology Group (ECOG) performance score (PS). The diagnosis of RA was established according to 1987 American College of Rheumatology (formerly American Rheumatism Association) revised classification criteria for RA (6) or 2010 American College of/European League against Rheumatism (ACR/EULAR) criteria (7). RA disease activity was assessed by CDAI (RA Clinical Disease Activity Index) (8). The systemic CTDs in our study were determined in the basis of ICD 10 M30–M36, including systemic scleroderma, systemic lupus erythematosus, Sjögren syndrome, RA, mixed connective tissue disease, undifferentiated connective tissue disease, polymyalgia rheumatica, Behçet’s disease, necrotizing vasculopathies, and dermatopolymyositis. Comorbid cardiovascular diseases were determined according to ICD 10 I20–I25 and I50, which include ischemic heart diseases and heart failure. Comorbid cerebrovascular diseases heart diseases were determined by ICD 10 I60–I69, which include intracerebral hemorrhage and cerebral infarction.
Chest radiological examinations were reviewed retrospectively by an independent reading of two chest radiologists in the absence of clinical information. The tumor located within 2 cm of the proximal bronchial tree, trachea, esophagus, major vessels, heart, pericardium, and other mediastinal structures is defined as the central lesion, while the one located larger than 2 cm of the aforementioned mediastinal structures is defined as the peripheral lesion. The peripherally located tumors were further grouped based on the lobe involved.
The current study was conducted in accordance with the guidelines of the Declaration of Helsinki and the ethical guidelines for epidemiological research and approved by the Institutional Review Board of Peking Union Medical Hospital (No. S-K253).
Statistical analysis
Continuous variables were summarized as mean values (SD) if normally distributed or as median (range) if skewed. Categorical variables were summarized as frequency and percentage. Continuous variables were compared using Student’s t-test or Manne-Whitney U test where appropriate. The Chi-squared test or Fisher’s exact test was used for nominal variables, and Manne-Whitney U test for ordinal variables. Clinicopathologic variables were tested with logistic regression in univariate and multivariate analyses. Statistical analysis was performed using the SPSS version 22.0 (SPSS, Chicago, IL, USA). A P<0.05 was considered to be statistically significant.
Results
Comparisons between the LC patients with RA and those without CTD
As shown in Table 1, the predominant histology type is adenocarcinoma in both groups. There were no statistically significant differences in the distribution of age, gender, smoking status, histology type, and tumor location between the two groups. However, a larger proportion of patients with stage IV disease was noted in LC with RA (59.1% vs. 39.2%, P=0.017). Besides, more LC patients with RA had an ECOG PS ≥2 (8.0% vs. 20.5%, P=0.015). On multivariate analysis controlling for age, gender, smoking history, tumor stage, presence of comorbid cardiovascular and cerebrovascular diseases and presence of RA, tumor stage (OR: 1.41, 95% CI: 1.23–13.70, P=0.021) and presence of RA (OR: 1.35, 95% CI: 1.34–11.16, P=0.012) demonstrated independent associations with poorer ECOG PS. In terms of anti-cancer treatment, there is significantly more patients received surgery (66.7% vs. 88.8%, P=0.013), radiotherapy (11.1% vs. 43.0%, P=0.010), and chemotherapy (38.9% vs. 66.4%, P=026) in stage I–III LC patients without CTD.
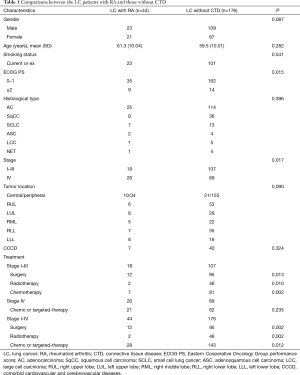
Full table
Clinicopathologic characteristics of LC patients with RA
Of the 44 LC patients with RA, 47.7% were female and 52.3% were male. The median age at LC diagnosis was 61 years (range, 32–86 years). RA onset preceded LC diagnosis by a median time of 9.5 years (range, 0.2–47 years). More than half (52.3%) of the patients were active smokers or former smokers, with a median smoking index of 12.5 pack-years (range, 8–80 pack-years).
As to RA disease activity, most patients are in remission. Only two patients showed low disease activity according to the values based upon CDAI, which did not affect the ECOG PS. Specific CT patterns of RA-interstitial lung disease (RA-ILD) were identified in 18 patients (40.9%), including usual interstitial pneumonia (UIP) pattern in 13 patients and non-specific interstitial pneumonia (NSIP) pattern in 5 patients. Results of serologic testing were not available in all LC patients with RA in the study; 31.7% (13/41) of the patients are ANA positive, 92.9% (39/42) RF positive, and 64.3% (27/42) CCP positive.
To assess the effect of smoking on LC patients with RA, we further compared the clinicopathological characteristics according to their smoking history (Table 2). A significant difference has been observed in the distribution of RA-ILD patterns. All patients with UIP have a smoking history, whereas NSIP was observed only in non-smoking patients (P<0.001). Besides, there is a significant difference in the ECOG PS scores at presentation between patients with and without a history of smoking (P=0.014). However, on multivariate analysis controlling for age, gender, smoking history, tumor stage, and presence of comorbid cardiovascular and cerebrovascular diseases, smoking history did not show an independent association with poorer ECOG PS (OR: 2.58, 95% CI: 0.75–233.31, P=0.078). No statistically significant differences were found in the distribution of tumor stage, location and histological type.
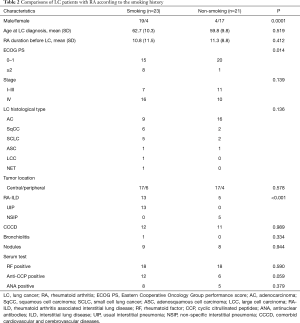
Full table
Anti-RA and anti-cancer treatment in LC patients with RA
Of the 44 LC patients with RA, 41 patients (93.2%) had received nonsteroidal anti-inflammatory drugs (NSAIDs); 23 (52.3%) had received glucocorticoids; 36 patients (81.8%) had received at least one disease-modifying anti-rheumatic drug (DMARD), including methotrexate in 29 patients, cyclophosphamide in 13 patients, leflunomide in 11 patients, and hydroxychloroquine in 9 patients. Besides, 23 patients have received Tripterygium glycosides, 27 patients received total glucosides of paeony. The information regarding the treatment history cannot be obtained in three patients.
As to antitumor treatment, 12 patients underwent curative surgery, 2 patients received adjuvant radiotherapy, 3 patients received EGFR-TKI, 25 patients received chemotherapy, including taxanes, pemetrexed, vinorelbine, gemcitabine, etoposide, and platinum, and 1 patient received anti-angiogenic treatment with bevacizumab in addition of chemotherapy. Among patients receiving chemotherapy, 7 underwent radical excision and received chemotherapy in the adjuvant setting, while the other 18 patients at an advanced stage received chemotherapy as the first-line treatment. In the first line setting, 7 out of 16 patients (43.8%) achieved partial response (PR) or stable disease (SD). Three patients received EGFR-TKI, and 2 achieved PR (all with EGFR sensitive mutation). No grade 3/4 adverse event was observed in patients undergoing radiotherapy, chemotherapy and targeted therapy. Among patients with RA-ILD who received chemotherapy or EGFR-TKI, no symptomatic or radiological exacerbation of ILD was observed.
Discussion
This study is the first cohort study comparing LC in patients with RA patients and those without CTD. Further, an intra-group comparison according to the smoking status in LC patients with RA was performed to assess the effect of smoking on LC patients with RA. Compared with LC patients without CTD, LC patients with RA were more likely to be diagnosed at an advanced stage and have a poorer ECOG PS score, and were less likely to receive surgery, radiotherapy, chemotherapy and targeted therapy.
As reported in the previous epidemiological study (9), male patients predominate in our control group of LC patients without CTD. In contrast, female patients account for nearly half of the cases in the LC with RA group, which might be attributed to the higher incidence of RA in women (10). Notably, only one female patient and 50% of total patients had a smoking history in the LC with RA group. Compared to the previously reported smoking rate in LC patients (11), the lower smoking rate in LC patients with RA suggested that RA itself, independent of tobacco exposure, may be a risk factor for LC. However, inter-group comparisons on gender ratio and smoking rate failed to show statistically significant differences, which is likely due to the limited sample size.
The most common location of LC is the upper lobes in both groups, which is in consistent with the previous epidemiologic study (12). The majority of LC patients with RA had adenocarcinoma, followed by squamous cell carcinoma (SqCC) and small cell carcinoma. A similar histological pattern of LC with CTD had been described in the reported literature, with a predominance of adenocarcinoma, and with few cases of SqCC and small cell carcinoma (13,14). As the underlying chronic inflammatory process in RA may participate in the development of LC, gene mutation profiling of LC in RA patients may be different from that in general population. However, a large proportion of patients in our study are diagnosed before the advent of routine molecular testing of LC, information regarding the EGFR or ALK mutation status was obtained in only 6 cases of adenocarcinoma, with 5 cases negative for both EGFR and ALK mutation, and 1 case positive for EGFR exon 21 L858R point mutation. Saijo et al. (15) reported that EGFR mutation was not observed in the 7 non-squamous NSCLC patients with CTD, whereas 10 out of 17 patients without CTD tested positive for EGFR mutations. Similarly, another study in systemic sclerosis showed that only 1/10 had EGFR mutation (16). However, interpretation of these data is limited by the small number of cases with molecular testing performed, and further comprehensive genomic profiling is warranted.
Unexpectedly, the majority of patients are diagnosed at an advanced stage in LC with RA group. This is at odds with our presumption that LC could be detected at an early stage in patients with RA thanks to a regular follow-up. A proposed explanation for the contradiction is that majority of the cases in our cohort are referred from rural hospitals where inadequate access to quality medical care often leads to a delayed diagnosis. Additionally, a selection bias may exist, given that RA patients with advanced LC are more likely to be referred to our center as RA associated comorbidities and the use of DMARDs greatly complicate the medical treatment for LC. Nonetheless, our findings raise concerns over the negligence of malignant pulmonary lesions in RA patients and highlight the need for increased attention to and efforts for optimization of cancer screening protocols in RA patients.
With regard to the ECOG scoring, a significantly larger proportion of LC patients with RA has an ECOG PS ≥2 compared to those without CTD. The comorbidities related to RA and its treatment, coupled with the more advanced stage of LC at presentation, may provide an explanation for the poorer performance status in LC patient with RA.
ILD has been reported to be the most common pulmonary manifestation in RA patients, occurring in 10–20% of patients (17). In our study, ILD was observed in 40.9% of LC patients with RA. A postulated reason for the higher prevalence of ILD in our study is that respiratory symptoms caused by LC may prompt a diagnostic imaging which could result in a recognition of either early ILD or very mild ILD that would otherwise be asymptotic. Of the 18 cases of RA-ILD in our study, 13 are UIP, while five are NSIP. In consistent, prior studies have also shown that although NSIP is the most common pattern in CTD-associated ILD, UIP is more frequent in RA-ILD (18,19). Notably, all cases with UIP were found in patients with smoking history, while NSIP was noted only in non-smoking patients. This suggested a role for smoking in the pathogenesis of UIP in RA patients. Besides, the presence of UIP was significantly more common in RA-associated LC compared with LC in general population. This could be easily explained as UIP is a common type of pulmonary involvement in RA (17).
With regard to the anti-cancer treatment, our study showed that LA patients with RA were less likely to receive surgery, radiotherapy, chemotherapy and targeted therapy. However, anti-cancer treatment is efficient and tolerated for LC patients with RA in this study, even in the presence of RA-ILD.
This study has several limitations, most notably its retrospective nature and single-institution design, which may introduce potential bias in patient selection and will limit the generalizability of our results. Another limitation is that the duration and dosage of DMARDs treatment could not be obtained which may contribute to the development of LC and ILD. Further, survival analysis should be performed, and an identification of potential risk factors would be of value. However, survival data cannot be attained in several patients in the study.
Conclusions
Compared with LC patients without CTD, LC patients with RA were more likely to be diagnosed at an advanced stage and have a poorer ECOG PS score, and were less likely to receive surgery, radiotherapy, chemotherapy and targeted therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of Peking Union Medical Hospital (No. S-K253) and written informed consent was obtained from all patients.
References
- Gabriel SE, Crowson CS, Kremers HM, et al. Survival in rheumatoid arthritis: A population-based analysis of trends over 40 years. Arthritis Rheum 2003;48:54-8. [Crossref] [PubMed]
- Turesson C, O’Fallon WM, Crowson CS, et al. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis 2003;62:722-7. [Crossref] [PubMed]
- Kauppi M, Pukkala E, Isomäki H. Excess risk of lung cancer in patients with rheumatoid arthritis. J Rheumatol 1996;23:1484-5. [PubMed]
- Buchbinder R, Barber M, Heuzenroeder L, et al. Incidence of melanoma and other malignancies among rheumatoid arthritis patients treated with methotrexate. Arthritis Rheum 2008;59:794-9. [Crossref] [PubMed]
- Parikh-Patel A, White RH, Allen M, et al. Risk of cancer among rheumatoid arthritis patients in California. Cancer Causes Control 2009;20:1001-10. [Crossref] [PubMed]
- Silman AJ. The 1987 revised American rheumatism association criteria for rheumatoid arthritis. Br J Rheumatol 1988;27:341-3. [Crossref] [PubMed]
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569-81. [Crossref] [PubMed]
- Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): A review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol 2005;23:S100-8. [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Symmons D, Turner G, Webb R, et al. The prevalence of rheumatoid arthritis in the United Kingdom: new estimates for a new century. Rheumatology 2002;41:793-800. [Crossref] [PubMed]
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [Crossref] [PubMed]
- Byers TE, Vena JE, Rzepka TF. Predilection of lung cancer for the upper lobes: An epidemiologic inquiry. J Natl Cancer Inst 1984;72:1271-5. [PubMed]
- Yang Y, Fujita J, Tokuda M, et al. Lung cancer associated with several connective tissue diseases: With a review of literature. Rheumatol Int 2001;21:106-11. [Crossref] [PubMed]
- Bonifazi M, Tramacere I, Pomponio G, et al. Systemic sclerosis (scleroderma) and cancer risk : systematic review and meta-analysis of observational studies. Rheumatology (Oxford) 2013;52:143-54. [Crossref] [PubMed]
- Saijo A, Hanibuchi M, Goto H, et al. An analysis of the clinical features of lung cancer in patients with connective tissue diseases. Respir Investig 2017;55:153-60. [Crossref] [PubMed]
- Katzen JB, Raparia K, Agrawal R, et al. Early stage lung cancer detection in systemic sclerosis does not portend survival benefit: A cross sectional study. PLoS One 2015;10. [Crossref] [PubMed]
- Yunt ZX, Solomon JJ. Lung disease in rheumatoid arthritis. Rheum Dis Clin North Am 2015;41:225-36. [Crossref] [PubMed]
- Adzić TN, Pesut DP, Nagorni-Obradović LM, et al. Clinical features of lung cancer in patients with connective tissue diseases: a 10-year hospital based study. Respir Med 2008;102:620-4. [Crossref] [PubMed]
- Lee HK, Kim DS, Yoo B, et al. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest 2005;127:2019-27. [Crossref] [PubMed]


