Surgical and endoscopic treatment for COPD: patients selection, techniques and results
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive inflammatory disease responsible for significant morbidity and mortality worldwide.
Smoking is a critical risk factor in the development and progression of the disease; even if all the smokers don’t develop COPD, nearly 90% of COPD patients are smokers.
Other causes of COPD can include occupational dusts and chemicals, alpha-1-antitrypsin deficiency, outdoor and indoor pollution, bronchial hypersensitivity.
The current management of the COPD is based on smoking cessation, bronchodilator therapy, anti-inflammatory drugs, antibiotics, mucolytics, antioxidants, prophylactic vaccination, pulmonary rehabilitation and home oxygen therapy.
Unfortunately, despite adequate medical treatment, a consistent amount of patients, especially those with advanced disease, continues to deteriorate quite rapidly failing to control the disease. Management of these patients includes a surgical approach like lung volume reduction surgery (LVRS) or in some cases lung transplantation.
The rationale behind LVRS was first described in 1957 by Brantigan and Mueller (1) but, due to the perioperative mortality rate of 18%, the surgical approach did not find fertile ground. After the study published by Cooper and colleagues in 1995 (2) that showed impressive clinical improvements in 20 cases of bilateral volume reduction surgery, the procedure is back in favour. However, published data regarding LVRS until 2003 essentially consisted of not randomized single-center case series including limited patient numbers and large variability in inclusion/exclusion criteria, type of surgery, duration of follow-up and interpretations of outcomes and morbidity.
Much of the information originates from the National Emphysema Treatment Trial (NETT), a multicenter prospective randomized controlled study published in 2003 that analyzed as primary endpoints the effect of LVRS on survival and functional performance on patients who received maximal medical treatment, including pulmonary rehabilitation, and patients who underwent maximal medical treatment in addition to LVRS. Secondary outcome measures included the influence of LVRS on pulmonary function tests, symptoms and quality of life.
NETT has implemented our knowledge about emphysema providing significant evidence that reducing hyperinflation in carefully selected patients can reduce mortality improving at the same time exercise capacity, lung function and quality of life.
Nevertheless, it appears nowadays that the potential of LVRS to be a disease-modifying therapy is not properly taken into account. The reasons for this are murky but may be due to an overwhelming skepticism about the utility of the procedure or an overestimation of mortality and morbidity rates.
A survey conducted by the British Thoracic Society showed that there is a significant information need about the indications for LVRS and the associated risks of morbidity and mortality. Besides that, there is a lack of multidisciplinary team able to discuss and establish the best approach for screening individuals to identify potential candidates for LVRS (3).
The introduction of minimally invasive techniques like the uniportal non-intubated awake procedure along with the development of endoscopic treatment have determined a renaissance of interest for this operation.
LVRS: indications, techniques and outcomes
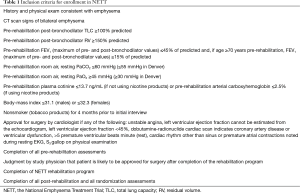
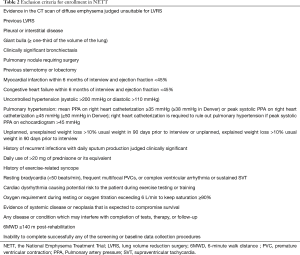
One of the most useful lesson learned from NETT is the careful choice of patients, possibly derived from the judgement of a multidisciplinary team, in order to exclude high risk patients favouring patients with severely symptomatic COPD who may improve after surgery with acceptable peri- and post-operative morbidity and mortality (Tables 1,2).
Full table
Full table
A careful medical history together with a high resolution computer tomography and body plethysmography with assessment of the diffusing capacity of the lung for carbon monoxide (DLCO) are the cornerstones of the pre-operative evaluation of COPD patients. LVRS should be considered in severe symptomatic patients with COPD who are not improving their clinical status despite maximal medical treatment including pulmonary rehabilitation. The presence of heterogeneous emphysema (upper lobe predominant) in a patient with severe obstruction (FEV1 ≤45% but >20% predicted), limited exercise capacity with hyperinflated lung are considered part of the standard criteria. An interesting definition introduced by NETT is the concept of patients with unacceptable high surgical risk like those with FEV1 ≤20% predicted and non-heterogeneous emphysema or DLCO ≤20% predicted. They have a reported mortality rate of 16% and therefore must be not considered candidate for surgery.
The unilateral distribution of emphysema with more severe destruction in a single lung has been considered as an elective indication for unilateral LVRS (4).
In opposition to previous studies, the NETT did not consider hypoxemia or need of supplemental oxygen as predictors of worse outcome.
The smoking cessation and the absence of ischemic heart disease, on the contrary, are conditio sine qua non to perform surgery without an increased morbidity and mortality rate. The cardiac assessment with a pharmacologic stress test and a coronary angiography (in case of high clinical suspicious or positive stress test) are recommended in order to rule out the presence of ischemic heart disease (5).
The aim of the LVRS is to exclude emphysematous and hyperinflated pulmonary areas thus decreasing the dead space, shifting the ventilation from poorly perfused zones to areas with a better perfusion and decreasing the V/Q mismatch. After removal of the hyperinflated lung, the normal compressed lung will expand (6). The video-assisted thoracic surgery (VATS) approach is nowadays considered the gold standard to perform LVRS in patients selected according to the above-mentioned criteria. The surgical technique presents no difficulties but the poor clinical status of the patients along with the fragile lung tissue make it a technical challenge potentially leading to several complications, especially prolonged air leak and respiratory failure.
Despite the increasing enthusiasm in the last years for the uniportal VATS, we believe that the conventional triportal approach is the safest option in order to avoid excessive retraction of the lung and subsequent tearing of the visceral pleura.
Even if there are to date no randomized studies that compare the unilateral versus the bilateral procedure, the one-stage bilateral LVRS is the most preferred approach.
The group of Leicester reported a staged bilateral approach with the timing of the second procedure dictated by the patient on the basis of their perception of symptomatic deterioration. This unique approach has led to good functional outcomes without jeopardise the survival (7).
The functional benefits on lung function and quality of life after bilateral LVRS have the optimal outcomes 3–6 months after surgery. The NETT Trial showed that only patients with upper-lobe predominant emphysema and a low base-line exercise capacity have an increased survival after surgery, but good functional improvements are reported in patients with upper-lobe emphysema and a high base-line performance status and in patients with non-upper-lobe emphysema and a low base-line performance status. The group of Ciccone reported an increase of the FEV1 6 months after surgery in 94% of cases along with an improvement of the DLCO of 25% compared to the preoperative values on follow up at 6 months and 1 year. Functional improvement was also measured by the 6-minute walking test (6MWT) with a reduction of dyspnea in 88% of patients at 6 months according to the Medical Research Dyspnea Scale (8). Gelb and colleagues showed an increase of FEV1 >200 mL in 88% of the patients after 6 months, respectively in 8% after 5 years and a survival of 96% at 6 months and 42% at 5 years (9).
The poor functional status of patients undergoing LVRS carries with it mortality and morbidity rates that are hard to ignore. Several studies reported a mortality rates between 0% and 17% (10-13) while the NETT showed an overall morbidity rate of 59% with major pulmonary and cardiovascular morbidity within 90 days after surgery occurring in 30% and 20% of patients, respectively. The most common complication was cardiac arrhythmia with a rate of 23.5%; pneumonia and need of reintubation occurred in 18% and 22% of cases, respectively. Other reported causes of morbidity include bleeding, respiratory failure, gastrointestinal complications, pulmonary hypertension, secondary pneumothorax, and the development of giant bullous emphysema.
DeCamp et al. showed in a study published in 2006 that within 30 days after surgery, about 90% of patients undergoing LVRS have an air leak lasting more than 30 days in 12% of cases. Risk factors for post-operative air leaks were lower DLCO, presence of upper lobe predominant disease and most importantly the presence of pleural adhesions. No advantage in air leaks duration was demonstrated with the use of various buttressing material including bovine pericardium (14).
Other studies showed a reduction of air leaks duration with the use of buttressed stapler without cost advantage or influence in hospital stay (15,16).
Even if pulmonary hypertension has been considered historically a contraindication for LVRS, a recent work of the group of Walter Weder in Zürich reported good functional outcomes with reduction of the median systolic pulmonary artery pressure from 41 mmHg [interquartile range (IQR), 39–47 mmHg] to 37 mmHg (IQR, 36–38 mmHg, P=0.04) in patients with heterogeneous emphysema and mild to moderate pulmonary hypertension (17).
In addition to the classical resectional VATS LVRS, several authors described a non resectional approach which provides for a plication of the most emphysematous lung region using a stapling, non-cutting device. This technique was first described in 1992 by Crosa-Dorado et al. (18) in patients who underwent a thoracotomy and subsequently adapted in VATS procedures in 1997 by Swanson (19). More recently, Pompeo et al., in a randomized study, showed that awake nonresectional LVRS could improve the rate of early discharges more than the non-awake resectional LVRS, without any differences in functional outcomes, survival or need of contralateral treatment for up to 36 months (20).
Quite apart from the type of the performed procedure, the cornerstone of the LVRS should be the gentle handling of the lung parenchyma, one of the essential condition to prevent post-operative air leak.
General anesthesia with double lumen endotracheal tube and single lung ventilation has been always considered the gold standard but, in recent years, there has been a growing interest among thoracic surgeons and anaesthesiologists in non-intubated awake procedures performed on lightly sedated patients under spontaneous ventilation. The non-intubated approach could potentially reduce adverse effects of intubation and ventilation related injuries (21). Preserving the diaphragm motion and the pulmonary compliance of the contralateral lung, which is more perfused during lateral decubitus, has a positive impact on the ventilation/perfusion match, reducing the risk of hypoxemia. In addition, avoiding endotracheal intubation and consequent mechanical ventilation reduce the risk of barotrauma into the friable lung parenchyma and therefore a potential source of post-operative air leak.
Equally important is the pain control: the effort focused on minimising the pain should be maximal! Patients with COPD have limited functional reserve; thus pain relief in the postoperative phase allows for active physiotherapy facilitating early recovery with positive effects on long-term outcomes. Several centers have developed a protocol in which the thoracic epidural anesthesia (TEA) or a paravertebral block is performed always pre or intraoperative in order to neutralize the latency period of all regional blocks.
Endoscopic lung volume reduction (ELVR): a new era is coming?
Despite the proven benefit of the LVRS, the heterogeneity of the COPD and the frailty of the patients have encouraged the search for new procedures to extend the treatment chance to more patients expanding therefore the eligibility criteria.
Several techniques including endobronchial valve, polymeric lung sealant, thermoablation and coil are available.
The use of endobronchial unidirectional valves (EBV) allows air to leave a pulmonary lobe or segment without enter it during the inspiratory phase inducing an atelectasis in the region distal to the valve. Consequently the volume of the emphysematous lung will be reduced with an improvement in pulmonary tests and exercise capacity in patients with hyperinflated lungs. Similarly to LVRS, the patient selection is essential to have successful outcomes. Even if there are no absolute spirometry cutoffs, the absence of collateral ventilation between the treated and ipsilateral lobes is the key point for procedural success. The presence of collateral ventilation should be assumed when the inter-lobar fissures are incomplete on high resolution computer tomography and should be confirmed by an objective method. Even if several software packages are available to try to determine the fissure completeness, the Chartis System is the most studied diagnostic tool to identify the absence of collateral ventilation in the target lung region. The ChartisTM Pulmonary Assessment System consists of a catheter placed through a bronchoscope with a balloon present at the distal end, which after inflation blocks the bronchus but allows air flow out from the target region only through the Chartis catheter’s central lumen. By connecting to a console, airway resistance and collateral ventilation can be measured in the isolated lung compartments.
Currently two types of valves are most investigated for clinical use: the Zephir valve and the intrabronchial valve (IBV). The first one (Pulmonx, Inc., Redwood City, CA, USA) has a self-expanding, membrane that opens during expiration and closes during inspiration. The IBV (Spiration, Inc., Tokyo, Japan) is an umbrella-shaped, one-way valve incorporating a nitinol skeleton consisting of five distal anchors holding the valve in place and six proximal struts covered by a polymer. Several side effect and complications are described after valve implantation: infections, COPD exacerbations, pneumonia, hemoptysis, valve migration/expectoration and pneumothorax. The last one is one of the most important complications of endobronchial valve placement with an incidence reported in the literature between 15% and 25%. The mechanism is thought to be a rapid shift in lung volumes caused by the rupture of subpleural bullae of the lobe close to the treated lobe after the induced atelectasis or the tearing of adhesions between the parietal and visceral pleura.
One of the first clinical trial on EBV, the Vent study, published by Sciurba et al. in 2010 showed unsatisfying results with a small increase in FEV1 of 6.8% and no differences between the valve group and the control group for the price of more frequent exacerbations of COPD, pneumonia, and hemoptysis after valve placement. The reported mortality was 3.7% in the EBV group at 12 months. Despite the disappointing results, the Vent study identified, with a subgroup analysis, a greater heterogeneity of emphysema and complete inter-lobar fissures as factors of clinical and physiological good responses to endobronchial-valve therapy (22). These last findings were showed later in several trials, like the LIBERATE or the TRANSFORM trial.
The TRANSFORM study, published in 2017, showed significant outcomes in pulmonary function, dyspnea, exercise performance, and quality of life, without increasing the mortality and morbidity rate. Ninety-seven patients were randomized: 65 assigned to EBV and 32 to standard of care (SOC). After 3 months, 55.4% of EBV and 6.5% of SOC subjects had an increase in FEV1 of 12% or more (P<0.001). Improvements were still present after 6 months: EBV 56.3% versus SOC 3.2% (P<0.001). Between-group differences for changes at 6 months were clinically significant: ΔEBV − SoC for residual volume, −700 mL; 6-minute-walk distance, +78.7 m; St. George’s Respiratory Questionnaire (SGRQ) score, −6.5 points; modified Medical Research Council dyspnea score, −0.6 points; and BODE (body mass index, airflow obstruction, dyspnea, and exercise capacity) index, −1.8 points (all P<0.05). Pneumothorax was the most common cause of morbidity, presenting in 29.2% of cases after EBV implantation (23).
The LIBERATE trial showed similar significant improvements in lung function, exercise tolerance, dyspnea and quality of life over standard medical therapy extended to at least 12 months (24).
Less studied are the IBV and thus the efficacy and safety profile for treatment of emphysema is limited by the small number of randomized controlled trials.
In order to bypass the problem of the collateral ventilation, the most important limiting factor for the use of the abovementioned valves, some authors suggested the use of sealants instead of endobronchial valves due to the alveolar level of action.
The use of biological sealant agents induces atelectasis due to airway occlusion and subsequent remodeling. This remodeling will cause an induced contraction of lung parenchyma with the loss of hyperinflated areas expected 1 to 2 months later. It should be taken in account that the use of sealing agents is not reversible and therefore the careful selection of patient and target site is essential. Although very promising, the long-term effect needs further investigation.
Similar to biological sealants, the treatment with thermal vapor ablation is not influenced by the collateral ventilation. A 2 mm vapor catheter can be inserted via bronchoscopy to targeted segmental airways producing an inflammatory response and, ultimately, lung volume reduction.
The benefits of this technique were analyzed in a recent multinational, multicenter randomized controlled trial [Sequential Staged Treatment of Emphysema with Upper Lobe Predominance (STEP-UP)] in patients with upper lobe predominant emphysema.
When compared to standard non-surgical management, the focused thermal vapor ablation of more hyperinflated areas resulted in improvements in lung function and quality of life after 6 months. The mean relative increase of the FEV1 between the treatment group versus the control group was 14.7% (95% CI, 7.8–21.5%; P<0.0001). COPD exacerbation was the most common complication, presenting in 11 (24%) of 45 patients in the treatment group and 1 (4%) of 24 in the control group. Remarkably, no patients experienced a pneumothorax within 30 days of treatment (25).
The irreversibility and the unpredictability of the inflammatory response of thermal vapor ablation as well as biological sealants limit their current use.
In contrast to the abovementioned techniques, the use of shape-memory nitinol coils seems to be effective also in homogeneous emphysematous lungs.
Three randomized controlled trials, the RENEW, the RESET and the REVOLENS, favoured the coil group over the control group (26-28). A recent metanalysis reported better outcomes in minimal clinically important difference (MCID) for FEV1 (RR =2.37, 95% CI, 1.61–3.48, P<0.0001), for 6MWT (RR =2.05, 95% CI, 1.18–3.53, P=0.01), and for SGRQ (RR =2.32, 95% CI, 1.77–3.03, P<0.00001) (29).
Conclusions
In the last 20 years, the knowledge and the amount of published data about lung volume reduction have increased significantly. What became clear more and more to date is the invaluable role of a multidisciplinary board in order to carefully select the best option for the right patient.
However, more randomized controlled trials comparing LVRS versus ELVR involving a large number of patients are needed with the aim of deepen long term outcomes, side effects and costs.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Brantigan OC, Mueller E. Surgical treatment of pulmonary emphysema. Am Surg 1957;23:789-804. [PubMed]
- Cooper JD, Trulock EP, Triantafillou AN, et al. Bilateral pneumectomy (volume reduction) for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg 1995;109:106-16; discussion 116-9. [Crossref] [PubMed]
- McNulty W, Jordan S, Hopkinson NS, et al. Attitudes and access to lung volume reduction surgery for COPD: a survey by the British Thoracic Society. BMJ Open Resp Res 2014;1. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Mineo D, et al. Results of unilateral lung volume reduction surgery in patients with distinct heterogeneity of emphysema between lungs. J Thorac Cardiovasc Surg 2005;129:73-9. [Crossref] [PubMed]
- Benzo R. Lung volume reduction surgery: non-pharmacological approach. Curr Opin Anaesthesiol 2011;24:44-8. [Crossref] [PubMed]
- Quezada W, Make B. Interventional Options for COPD- LVRS, Bronchoscopic Therapies and the Future. Chronic Obstr Pulm Dis 2016;3:446-53. [Crossref] [PubMed]
- Oey IF, Morgan MD, Spyt TJ, et al. Staged bilateral lung volume reduction surgery - the benefits of a patient-led strategy. Eur J Cardiothorac Surg 2010;37:846-52. [Crossref] [PubMed]
- Ciccone AM, Meyers BF, Guthrie TJ, et al. Long-term outcome of bilateral lung volume reduction in 250 consecutive patients with emphysema. J Thorac Cardiovasc Surg 2003;125:513-25. [Crossref] [PubMed]
- Gelb AF, McKenna RJ Jr, Brenner M, et al. Lung function 5 yr after lung volume reduction surgery for emphysema. Am J Respir Crit Care Med 2001;163:1562-6. [Crossref] [PubMed]
- Criner GJ, Cordova FC, Furukawa S, et al. Prospective randomized trial comparing bilateral lung volume reduction surgery to pulmonary rehabilitation in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:2018-27. [Crossref] [PubMed]
- Goldstein RS, Todd TRJ, Guyatt G, et al. Influence of lung volume reduction surgery (LVRS) on health related quality of life in patients with chronic obstructive pulmonary disease. Thorax 2003;58:405-10. [Crossref] [PubMed]
- Miller JD, Malthaner RA, Goldsmith CH, et al. A randomized clinical trial of lung volume reduction surgery versus best medical care for patients with advanced emphysema: a two-year study from Canada. Ann Thorac Surg 2006;81:314-20; discussion 320-1. [Crossref] [PubMed]
- Hillerdal G, Lofdahl CG, Strom K, et al. Comparison of lung volume reduction surgery and physical training on health status and physiologic outcomes: a randomized controlled clinical trial. Chest 2005;128:3489-99. [Crossref] [PubMed]
- DeCamp MM, Blackstone EH, Naunheim KS, et al. Patient and surgical factors influencing air leak after lung volume reduction surgery: lessons learned from the national emphysema treatment trial. Ann Thorac Surg 2006;82:197-206; discussion 206-7. [Crossref] [PubMed]
- Hazelrigg SR, Boley TM, Naunheim KS, et al. Effect of bovine pericardial strips on air leak after stapled pulmonary resection. Ann Thorac Surg 1997;63:1573-5. [Crossref] [PubMed]
- Stammberger U, Klepetko W, Stamatis G, et al. Buttressing the staple line in lung volume reduction surgery: a randomized three-center study. Ann Thorac Surg 2000;70:1820-5. [Crossref] [PubMed]
- Caviezel C, Aruldas C, Franzen D, et al. Lung volume reduction surgery in selected patients with emphysema and pulmonary hypertension. Eur J Cardiothorac Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Crosa-Dorado VL, Pomi J, Perez-Penco EJ. Treatment of dyspnea in emphysema pulmonary remodeling: hemo and pneumostatic suturing of the emphysematous lung. Res Surg 1992;4:1-4.
- Swanson SJ, Mentzer SJ, DeCamp MM Jr, et al. No-cut thoracoscopic lung plication: a new technique for lung volume reduction surgery. J Am Coll Surg 1997;185:25-32. [Crossref] [PubMed]
- Pompeo E, Rogliani P, Tacconi F, et al. Randomized comparison of awake nonresectional versus nonawake resectional lung volume reduction surgery. J Thorac Cardiovasc Surg 2012;143:47-54, 54.e1.
- Minervini F, Bertolaccini L, Patrini D, et al. Non intubated awake uniportal VATS: how to start? Video-Assisted Thoracic Surgery 2018. In Press. [Crossref]
- Sciurba FC, Ernst A, Herth F, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233-44. [Crossref] [PubMed]
- Kemp SV, Slebos DJ, Kirk A, et al. A multicenter randomized controlled trial of Zephyr endobronchial valve treatment in heterogeneous emphysema (TRANSFORM). Am J Respir Crit Care Med 2017;196:1535-43. [Crossref] [PubMed]
- Criner GJ, Sue R, Wright S, et al. A Multicenter RCT of Zephyr® Endobronchial Valve Treatment in Heterogeneous Emphysema (LIBERATE). Am J Respir Crit Care Med 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Herth FJ, Valipour A, Shah PL, et al. Segmental volume reduction using thermal vapour ablation in patients with severe emphysema: 6-month results of the multicentre, parallel-group, open-label, randomised controlled STEP-UP trial. Lancet Respir Med 2016;4:185-93. [Crossref] [PubMed]
- Sciurba FC, Criner GJ, Strange C, et al. Effect of Endobronchial Coils vs Usual Care on Exercise Tolerance in Patients With Severe Emphysema: The RENEW Randomized Clinical Trial. JAMA 2016;315:2178-89. [Crossref] [PubMed]
- Shah PL, Zoumot Z, Singh S, et al. Endobronchial coils for the treatment of severe emphysema with hyperinflation (RESET): a randomised controlled trial. Lancet Respir Med 2013;1:233-40. [Crossref] [PubMed]
- Deslée G, Mal H, Dutau H, et al. Lung Volume Reduction Coil Treatment vs Usual Care in Patients With Severe Emphysema: The REVOLENS Randomized Clinical Trial. JAMA 2016;315:175-84. [Crossref] [PubMed]
- Wang Y, Lai TW, Xu F, et al. Efficacy and safety of bronchoscopic lung volume reduction therapy in patients with severe emphysema: a meta-analysis of randomized controlled trials. Oncotarget 2017;8:78031-43. [PubMed]


