(J)ALEX the great: a new era in the world of ALK inhibitors
Anaplastic lymphoma kinase (ALK) gene rearrangement defines a recently identified molecular subtype of non-small cell lung cancer (NSCLC), which accounts for 4–5% of all non-squamous NSCLC (1). ALK gene rearrangement was shown to be reliably diagnosed by either a fluorescence in situ hybridization (FISH) assay directly showing the result of 2p chromosome inversion, with the use of break-apart probes, by reverse transcription PCR detecting ALK fusion transcript, or using immunohistochemistry with a specific monoclonal anti-ALK antibody, showing the tumor cell accumulation of the fusion protein encoded by the new gene generated by such rearrangements (2). In NSCLC, the dominant 5' fusion partner is EML4, with other rarer partners such as KIF5B, all encoding for a coil-coiled domain responsible for the fusion protein homo-dimerization, leading to the constitutive ALK tyrosine kinase trans-phosphorylation and activation (3). Four years after its first identification in 2007, a retrospective study comparing the 82 patients included in the first phase I trial assessing the ALK tyrosine kinase inhibitor crizotinib, and 36 ALK-positive patients who did not receive crizotinib, showed 74% 1-year and 54% 2-year overall survivals in patients who were given crizotinib in second or third line settings, but only 44% and 12% 1- and 2-year OS respectively, in the 36 ALK-positive control patients given any second-line therapy (4). This retrospective study did suggest the activity of crizotinib and supported the lack of favorable prognostic impact for ALK rearrangement in advanced NSCLC. The phase I trial, up-dated in 2012 (5), then reported the striking effect of the tyrosine-kinase inhibitor crizotinib on progression-free and overall survival of stage IV pre-treated patients, whose tumor exhibited such a gene rearrangement. A 60% overall response rate (ORR) was reported in these patients with a median PFS of 9.7 months and an estimated 12-month PS of 74.8%.
The exquisite antitumor activity of crizotinib was further established in a randomized phase 3 trial (Profile 1007), comparing in 347 patients with advanced ALK-positive NSCLC who had received one prior platinum-based chemotherapy, either second-line crizotinib or second-line chemo (pemetrexed or docetaxel) (6). The median PFS was 7.7 months in the crizotinib group and 3.0 months in the chemotherapy group (HR =0.49; 95% CI, 0.37–0.64]; P<0.001) (Table 1). Finally the registration phase 3 PROFILE 1014 trial demonstrated the superiority of first-line crizotinib over platinum-based chemotherapy in treatment-naive ALK positive NSCLC patients (7). Indeed, ORR were 74% and 45%, respectively (P<0.001) and PFS was significantly longer with crizotinib than with chemotherapy (median, 10.9 vs. 7.0 months; HR =0.45; 95% CI, 0.35–0.60; P<0.001) (summarized in Table 1). Since crossover to crizotinib treatment after disease progression was permitted for patients receiving chemotherapy, overall survivals did not differ significantly between the two treatments, but still, crizotinib was considered as the standard of care for front-line treatment in ALK-positive patients. However, it rapidly appeared that, despite initial responses to crizotinib, the majority of patients have a systemic relapse within 12 months, owing to acquired resistance mutations within the ATP-binding domain of the ALK-kinase enzymatic site, some of them being homologous to the T790M gatekeeper mutation in EGFR (12,13). A large spectrum (around 20 different mutations) of resistance mutations have been described either in patients or using in vitro cell lines screens. Various other mechanisms leading to the activation of alternative signaling pathways (ALK-independent mechanisms) have been also described such as alternate oncogene activations (14). Also, in some situations progression only occurs in the central nervous system due to a poor diffusion of crizotinib in brain tissue.
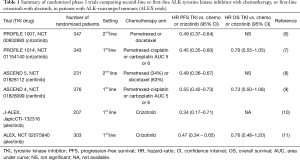
Full table
Despite the diversity of resistance mechanisms, most crizotinib-resistant tumors, especially those with secondary resistance mutations, remain ALK-dependent and are sensitive to more potent, structurally distinct, new second-generation ALK inhibitors, the first developed being ceritinib. After a first phase 1 study showing the activity of oral ceritinib in patients harboring ALK rearrangement, and who, for some, had previously received crizotinib, with a 56% response rate (15,16), the ASCEND 5 randomized phase 3 trial demonstrated the superiority of ceritinib over pemetrexed or docetaxel, in patients previously treated by crizotinib and platinum-based doublet chemotherapy (Table 1). Indeed, ceritinib showed a significant improvement in median PFS compared to chemo (5.5 months for ceritinib versus 1.6 months, HR =0.49, 95% CI, 0.36–0.67]; P<0.0001) (8). Frontline ceritinib was further tested versus platinum-based chemotherapy in the ASCEND 4 randomized phase 3 trial (see Table 1), and also showed a significant increase of median PFS (16.6 versus 8.1 months, HR =0.55, 95% CI, 0.42–0.73, P<0.001), while estimated 2-year survival was 70.6% compared with 58.2% for chemotherapy (HR =0.73, 95% CI, 0.50–1.08, P=0.056) although 60% of the patients in the chemo group had received an ALK-inhibitor since a cross-over was allowed in this trial (9). A major limitation in the wide use of ceritinib is certainly its relatively frequent GI toxicity, especially nausea and vomiting, possibly due to the huge size of the pills, which clearly alters quality of life in real-life patients. Then came alectinib, another second-generation class ALK inhibitor of which clinical development was truly innovative compared with previous ALK inhibitors. Indeed, clinical activity was first proved in a U.S. dose-finding phase 1/2 trial also showing a selective activity of alectinib on brain metastases, in patients who had previously been treated by crizotinib (17). Then, a North-American phase 2 trial in 87 ALK-positive patients progressing after crizotinib treatment, showed a 48% ORR, with a favorable safety profile (18). And finally, two parallel randomized phase 3 trials were launched comparing in Asian (10) and Caucasian (11) populations respectively, face to face, frontline crizotinib to frontline alectinib in ALK inhibitor-naive ALK-positive patients (summarized in Table 1). This courageous design was successful since both trials turned out to meet their primary endpoint, showing a significant PFS advantage for alectinib compared with crizotinib, also supporting a better efficacy in preventing brain metastasis evolution.
Specifically J-ALEX is the first trial to directly compare two ALK inhibitors, in ALK inhibitor first-line setting. It was a company-sponsored (Chugai Pharmaceutical Co., Ltd.), multicenter Japanese trial (10). Patients with untreated non-symptomatic brain metastasis at diagnosis were allowed, and actually represented overall 21% of patients with twice more brain metastasis patients in the crizotinib arm (n=31 vs. 16). Stratification factors were ECOG performance status (0 or 1 vs. 2), treatment line (first or second, after platinum-based chemotherapy), and disease stage (wet IIIB or IV vs. postoperative recurrence). The primary endpoint was PFS, as assessed by an independent facility, with a non-inferiority hypothesis (and a non-inferiority margin on the HR scale as 1.2). If the non-inferiority null hypothesis was rejected, a second superiority hypothesis could be tested, according to a hierarchical hypothesis testing procedure. And finally, overall survival analyses had only to be done, if the null hypothesis for the superiority in progression-free survival was rejected, owing that an interim analysis could be performed after 33% (55 events), by an independent data monitoring committee (IDMC), using O’Brien and Fleming-type alpha spending functions, for progression-free survival and overall survival. During a short 19-months accrual period, 207 patients were enrolled, and randomly assigned to receive alectinib (n=103) or crizotinib (n=104). As the upper confidence limit of HR for progression-free survival was lower than pre-defined non-inferiority margin 1.2, the superiority hypothesis could be tested and indeed, after a median follow-up of 12.0 months, median progression-free survival was significantly prolonged with alectinib (not estimable 95% CI, 20.3–NE) compared with crizotinib: 10·2 months (8.2–12.0); HR =0.34 (99.7% CI, 0.17–0.71); stratified log-rank test P<0.0001 with overall 40% progression-free survival events (24.3% in the alectinib group, 55.7% in the crizotinib group). The superiority trend in terms of progression-free survival of alectinib over crizotinib was consistent across most predefined patient subgroups (first-line setting versus second-line setting, IIIB–IV stage versus postoperative relapses), leading to an unprecedented projected median PFS over 2 years! More strikingly, the ORR as assessed by IRF was an amazing 91.6% rate, 95% CI, 85.6–97.5) for alectinib compared with 70.2% 95% CI, 61.4–79.0) for crizotinib which is already quite good.
The safety analysis did clearly favor alectinib, since the total number of patients with at least one grade 3 or 4 adverse event was higher in the crizotinib group [54 (52%) of 104] than in the alectinib group [27 (26%) of 103]. Furthermore, dose interruptions due to adverse events were more frequent with crizotinib [77 (74%) of 104] than alectinib [30 (29%) of 103], and more patients receiving crizotinib discontinued study drug due to an adverse event [21 (20%)] than those receiving alectinib [9 (9%)]. Overall survival data in both groups were still immature since the IDMC recommended immediate release of the trial results, based on PFS data. However, one of the most striking finding of this trial is the preplanned exploratory analysis of cumulative incidence of CNS progression and non-CNS progression events, indicating that alectinib remarkably reduced the risk of progression in both non-CNS and CNS lesions compared with crizotinib. Such significant trend was confirmed in the Caucasian randomized phase 3 trial ALEX, with a comparable design, which was reported several months after J-ALEX (11), and in which the 12-months cumulative incidence of brain metastases was 41.4% in the crizotinib versus 9.4% in the alectinib arm (19). However in the ALEX trial, patients were enrolled in 31 countries with a majority of Caucasian patients, they were true treatment-naive patients since first-line chemotherapy was not allowed, and they received 600 mg alectinib twice daily compared to 300 mg twice daily, the “Asian dose” in J-ALEX. The same rate of measurable CNS disease was observed in both trials, around 13–14% but in the ALEX trial, CNS metastatic patients accounted for 42% and 38% of the patients in the alectinib group and the crizotinib group respectively, without any unbalance as encountered in J-ALEX. The confirmation of the remarkable safety profile of alectinib, even at the Caucasian dose, has also been provided by ALEX trial, since especially GI toxicity clearly appeared less frequent and of lower grades than historical data with ceritinib.
Around 24 months after the first release of these results, which founded the U.S. and European alectinib registration, while alectinib is not widely available, notably in some European countries since its price has to be set in each European country by specific negotiations between the company and the local health authorities, could we consider that alectinib should be considered as the reference frontline treatment for ALK-positive metastatic patients? While the question of the application of Japanese data could be expended to a broader Caucasian population has been clearly answered by the ALEX trial, the remaining question is the preferred strategy: either first-line first generation anti-ALK drug (crizotinib), followed at progression by a second-generation drug (alectinib rather than ceritinib because of the better GI tolerance), and then by other newer anti-ALK drugs such as brigatinib or lorlatinib, or frontline second-generation anti-ALK drug (alectinib) followed by other newest drugs. Since we do not have OS data from the ALEX trials, because of their immaturity, it is virtually impossible to definitively answer this question. However, these data would not change the figure. First, with a median follow-up ranging from 17 to 20 months, the impressive observed PFS data, with projected median values exceeding 2 years, are highly suspected to translate into OS benefits compared to historical controls who are the first patients treated with crizotinib and who did not benefit from second-line generation drugs. Second, the OS mature data from the ALEX trials will be difficult to interpret since a majority of patients will be able to receive other ALK-inhibitors, ceritinib, brigatinib and lorlatinib, possibly taking into account for the re-biopsy data and the precise nature of the resistance molecular event occurring at progression. Third, a major issue is that such patients should also be exposed to pemetrexed-platinum-based chemotherapy of which efficacy is high in ALK-positive population. And finally, recent data from IMPOWER 150 trial presented at 2018 AACR meeting in Chicago, assessing first-line chemotherapy plus immuno-oncology (i.o.) drug atezolizumab and the anti-VEGF monoclonal antibody bevacizumab, showed encouraging results in the ALK subgroup, although limited in size (n=34 patients). Thus, it is virtually impossible to ascertain that patients of both ALEX trials would have received all possible lines of therapy, without any unbalance, leading to a valuable comparison in the OS data of both crizotinib-first or alectinib-first arms. Accordingly, a notable number of patients rapidly progress with rapid general condition deterioration precluding any second-, third- or fourth-line therapy. This attrition is the support of a general aphorism never contradicted in the whole history of cancer treatments, especially of lung cancer treatments (because the course of this disease is rarely indolent): “the most effective drug should be always given first!”.. to avoid the risk not to be received in case of rapid patient general deterioration when disease progresses if not prescribed in frontline.
Alectinib was reported to be active (at least on in vitro cell lines models or patient-derived xenograft mice models), on G1123S, L1152P/R, C1156Y/T, F1174C/L/V, L1196M, S1206C/Y, G1269A/S crizotinib-induced resistance mutations (20,21), while remaining ineffective on 1151Tins, I1171T/N, V1180L, L1198F, G1202R resistance mutations (22) most of them being effectively targeted by the newest brigatinib and third-generation drug lorlatinib (23). Additionally, in vitro data, supported by whole exome sequencing data on repeat biopsies from lorlatinib-resistant patients, suggested that sequential ALK inhibitors could foster the emergence of compound ALK mutations (24). Indeed, accelerated ENU mutagenesis screening of Ba/F3 cells expressing EML4-ALK failed to induce the emergence of lorlatinib-resistant clones, while the same methodology used with cells expressing EML4-ALK and a single crizotinib-resistance mutation, lead to the occurrence of lorlatinib-resistant clones, of which 2 were found in patients’ repeat biopsies. Thus, these data could support the use of the most active drug frontline without previous exposure to first-generation drug crizotinib. Whether third-generation drug lorlatinib would be more active than alectinib, remains questionable, since the only major crizotinib-induced secondary mutation not covered by alectinib, but efficiently targeted by lorlatinib is the G1202R mutation. Unless a face-to-face randomized trial directly comparing frontline lorlatinib to alectinib we will not have easily the answer. What will be the secondary mutations emerging after frontline alectinib therapy (without crizotinib exposure) is not perfectly known yet, some observations reporting new alectinib-induced gatekeeper resistance mutations, that could be overcome by ceritinib, emphasizing again the need to rebiopsy progressing patients and to analyze precisely which molecular event drives the progression, since here, a role for sequential therapy with multiple next-generation ALK-TKIs could emerge (22). And finally some lorlatinib-induced resistance mutation could resensitize ALK-positive tumor cells to crizotinib suggestion the possibility of a rotation in the use of ALK inhibitors (25).
To conclude, ALEX trials did really change the panorama of research in ALK-driven lung cancer, by abandoning chemotherapy as the obliged comparator for evaluation of new ALK inhibitors, and by demonstrating the exquisite efficacy of alectinib on brain metastases. Those trials also remind us the need to re-biopsy patients, if possible in the most recently progressing tumor site (not always the initial primary site), to correctly assess which resistance mechanism is involved, since we have, now, many efficient drugs in this disease, including pemetrexed-platinum chemotherapy plus or minus i.o., in case of ALK-independent resistance mechanism, the sequential lines leading to improve survival compared to historical controls.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. G. Zalcman and V. Gounant reported personal fees and non-financial support from ROCHE and PFIZER. Dr. S. Brosseau has no relationships that present a potential conflict of interest to disclose.
References
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Martelli MP, Sozzi G, Hernandez L, et al. EML4-ALK rearrangement in non-small cell lung cancer and non-tumor lung tissues. Am J Pathol 2009;174:661-70. [Crossref] [PubMed]
- Choi YL, Takeuchi K, Soda M, et al. Identification of novel isoforms of the EML4-ALK transforming gene in non-small cell lung cancer. Cancer Res 2008;68:4971-6. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 2011;12:1004-12. [Crossref] [PubMed]
- Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012;13:1011-9. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385-94. Erratum in: N Engl J Med 2015;373:1582. [Crossref] [PubMed]
- Solomon BJ, Mok T. First-line crizotinib in ALK-positive lung cancer. N Engl J Med 2015;372:782. [PubMed]
- Shaw AT, Kim TM, Crinò L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017;18:874-86. [Crossref] [PubMed]
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. Erratum in: Lancet 2017;389:908. [Crossref] [PubMed]
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017;390:29-39. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 2012;18:1472-82. [Crossref] [PubMed]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Costa DB, Kobayashi S. Acquired resistance to the ALK inhibitor crizotinib in the absence of an ALK mutation. J Thorac Oncol 2012;7:623-5. [Crossref] [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Kim DW, Mehra R, Tan DSW, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol 2016;17:452-63. [Crossref] [PubMed]
- Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014;15:1119-28. [Crossref] [PubMed]
- Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234-42. Erratum in: Lancet Oncol 2017;18:e134. [Crossref] [PubMed]
- Gadgeel SM, Shaw AT, Govindan R, et al. Pooled Analysis of CNS Response to Alectinib in Two Studies of Pretreated Patients With ALK-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:4079-85. [Crossref] [PubMed]
- Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med 2012;4. [Crossref] [PubMed]
- Zhang S, Wang F, Keats J, et al. Crizotinib-resistant mutants of EML4-ALK identified through an accelerated mutagenesis screen. Chem Biol Drug Des 2011;78:999-1005. [Crossref] [PubMed]
- Katayama R, Friboulet L, Koike S, et al. Two novel ALK mutations mediate acquired resistance to the next-generation ALK inhibitor alectinib. Clin Cancer Res 2014;20:5686-96. [Crossref] [PubMed]
- Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 2017;18:1590-9. [Crossref] [PubMed]
- Yoda S, Lin JJ, Lawrence MS, et al. Sequential ALK Inhibitors Can Select for Lorlatinib-Resistant Compound ALK Mutations in ALK-Positive Lung Cancer. Cancer Discov 2018;8:714-29. [Crossref] [PubMed]
- Shaw AT, Friboulet L, Leshchiner I, et al. Resensitization to Crizotinib by the Lorlatinib ALK Resistance Mutation L1198F. N Engl J Med 2016;374:54-61. [Crossref] [PubMed]


