Complications related to endoscopic lung volume reduction for emphysema with endobronchial valves: results of a multicenter study
Introduction
Emphysema is a leading cause of morbidity and mortality. Lung transplantation is the only curative treatment but the strict eligibility criteria and an inadequate supply of donor organs make it available only in a limited number of patients. Lung volume reduction surgery (LVRS) has been proposed as a viable option for patients with heterogeneous emphysema. Despite the positive clinical results, LVRS is associated with significant morbidity (20–30%) and high 90 days operative mortality (7.9%) (1). Thus, over the years alternative minimally invasive techniques have been explored as the implantation of non-blocking (i.e., coil, sealant, vapor) or blocking devices [endobronchial valves (EBV)] to obtain bronchoscopic lung volume reduction (BLVR). Among these, EBV are the best studied to date. EBVs are designed to achieve BLVR by inducing lobar atelectasis. Exclusion of the most destroyed emphysematous lobe with EBV allows air to exit during expiration, but stops it from entering during inspiration, thus resulting in atelectasis of the target lobe and reducing hyperinflation. Heterogeneous emphysema and the absence of interlobar collateral ventilation (CV-negative patients) are the main predictive factors for the success of the procedure. EBV treatment is a well-tolerated procedure that provides clinically significant results in selected cases, but it can be associated with different complications that are challenge to treat due to poor clinical conditions of patients (2-8). Most of the papers published focused on the clinical results of EBV treatment while the incidence and management of complications related to EBV treatment remain less explored issue. The aim of the present paper is to evaluate the rate and type of complications related to EBV treatment and their impact on clinical outcomes.
Methods
Study design
This is a retrospective multicenter study including 8Italian centers with long experience in EBV treatment. All consecutive patients with heterogeneous emphysema undergoing BLVR with Zephyr EBVs from January 2012 to May 2017 and developing any type of complication related to the procedure were considered. Patients with lack of data regarding the type of complications and their treatment, and/or with incomplete follow-up were excluded. The goals of the paper were (I) to evaluate the incidence and the management of complications related to the procedure and (II) whether these complications could affect clinical outcome. Being it a retrospective study, the management of complications and the timing of follow-up were not standardized but decided by each participating center according to personal experience and guidelines. The study design was approved by the Ethic Local Committee of the two Coordinating Centers (University of Campania “Luigi Vanvitelli” and Sapienza University-Rome Sant’ Andrea Hospital). Patients signed a written informed consent for the EBV treatment and they were aware that their data could be anonymously analyzed for scientific purposes only. As the data in this current analysis were retrospectively analyzed, no further patients consent was required.
Study population
Patients were scheduled for EBV treatment according to the published best practice criteria (9-17). All patients had a diagnosis of severe emphysema (GOLD stage III or IV) and a residual volume (RV) ≥150% predicted. Emphysema distribution was assessed by high resolution computed tomography (HRCT) scan with volume rendering and lung perfusion scan; only lobes showing a clear density reduction without perfusion were treated (9,10). According to standard clinical practice (11-13), only patients considered as CV-negative for completeness of fissure ≥90% on HRCT-analysis and/or for the absence of CV at Chartis assessment were treated. Pulmonary function tests (PFTs) included forced expiratory volume in one second (FEV1), forced vital capacity (FVC), total lung capacity (TLC), RV, diffusing capacity (DLCO), 6-minute walking test (6MWT), PaO2 and PaCO2 (measured at rest while breathing room air). All pulmonary function data were presented as a percentage of predicted values for the patient’s age, gender and height. Quality of life was measured by the St. George’s Respiratory Questionnaire (SGRQ), ranging from 0 to 100, with a higher score indicating a worse quality of life. After treatment, all patients underwent standard clinical and radiological follow-up performed 3, 6, 12 months after the valves implantation, and then yearly.
Procedure of valve implant
The type of anesthesia used during the procedure varied across the centers, depending on patient condition and physician preference. Generally, the procedure was performed with IV sedation, spontaneous breathing, and flexible bronchoscopy. Alternatively, general anesthesia with either a laryngeal mask or endotracheal tube or rigid bronchoscopic to provide positive airway pressure or intermittent negative pressure ventilation was used. Three different size of valves were used: EBV 4.0 or EBV 4.0 LP (shorter), and EBV 5.5 for bronchial lumens with diameters of 4.0–7.0 mm and of 5.5–8.5 mm, respectively. After estimating the size of the target bronchus with a dedicated catheter, the valves were delivered through the same catheter to obtain the complete occlusion of the target lobe. All patients were hospitalized for a minimum of 48–72 hours after the procedure. A chest X-ray was performed few hours later or the day after the procedure according to the center’s experience, to assess the lobar volume reduction and to determine EBV location and to evaluate the presence of pneumothorax or other complications. In presence of any complications, additional diagnostic exams and treatment were carried out.
Morbidity and mortality
The type of complications, the time of onset from EBV implant (hour or days), the treatment and clinical and functional out-come were analyzed. In agreement with previous studies, the clinical response to treatment was assessed using the principle of minimal clinically important differences (MCID) that was defined according to the following specific responders criteria: an improvement of ≥15% in FEV1; an improvement of −8% in RV; an improvement of ≥26 m in 6MWT; an improvement of ≥4 points on the SGRQ. A target lobe volume reduction (TLVR) ≥350 mL defined the significant cut-off criterion for lung volume reduction.
Statistical analysis
Data were expressed as means ± standard deviation (SD) for continuous variables and absolute number and percentage for categorical variables. The delta for each variable was calculated by the variation between the value of the last follow-up and the baseline value. The difference between the different groups were calculated using Student’s t-test for quantitative variables and Chi-square test for categorical variable. A P value <0.05 was considered statistical significant. MedCalc statistical software (version 12.3, Broekstraat 52; 9030 Mariakerke; Belgium) was used for the analysis.
Results
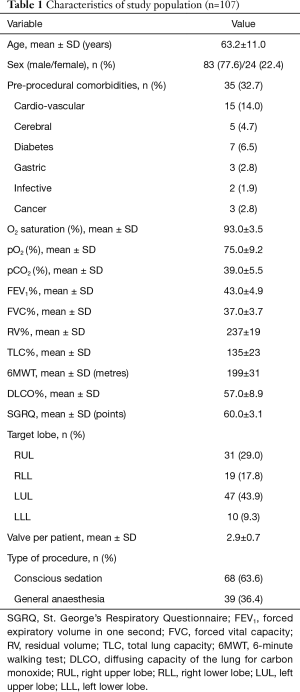
In the study period 423 patients underwent EBV treatment for severe emphysema. Of these, 107 (25.3%) patients developed EBV-related complications and were included in the study. The characteristics of the study population are summarized in Table 1. Mean age was 63.2±11.0 years old and most of patients were male (77.6%). Thirty-five patients (32.7%) presented pre-procedural co-morbidities. Before treatment, the mean value of FEV1% and of RV% were 43.0±4.9 and 237±19, respectively. The left upper lobe (LUL) was the most treated lobe (43.9%). The mean number of valves per patient were 2.9±0.7 and most of the procedures were performed with conscious sedation (63.6%).
Full table
Complications related to valves
The type of complications, treatment and outcome are summarized in Table 2. During the study period 5 out of 423 (1.2%) treated patients died 27.0±3.9 months from treatment. In all cases the cause of death was not related to valves treatment. Two patients died for lung and brain cancer, respectively, one for stroke, one for myocardial ischemic attack, and one for drug-induced anaphylaxis.
Full table
Pneumothorax
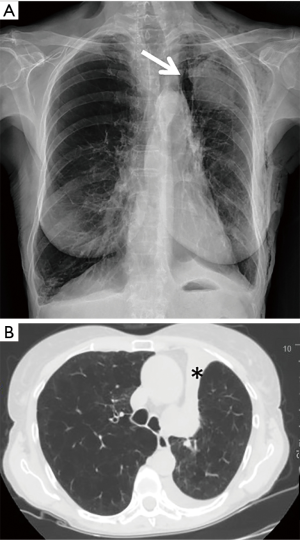
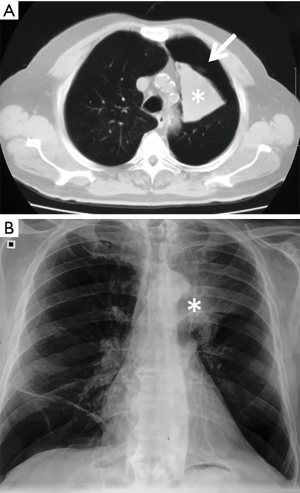
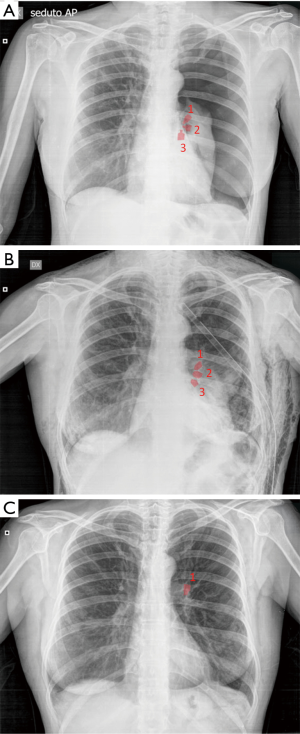
Pneumothorax was the most common complication, observed in 73 out of 423 (17.3%) treated patients. Among these, 54/73 (74.0%) patients had a TLVR >350 mL. In 86.3% (63/73 patients) of cases pneumothorax occurred during hospitalization (22.0±9.3 hours after valve implant) and in 13.7% (10/73 patients) pneumothorax occurred after a mean time of 5.3±2.8 months. The treatment included clinical observation in 23 (31.5%) cases (Figure 1); chest drainage in 38 (52.1%) cases for the presence of massive pneumothorax (Figure 2), and chest drainage with valve(s) removal in 12 (16.5%) cases for persistent air leaks (more than 5 days) (Figure 3). In all cases the pneumothorax successfully resolved after a mean time of 4.0±1.9 days. Eight of 12 (66.7%) patients, in whom the valves were removed, underwent re-implantation of the valves and TLVR >350 mL was obtained in seven of these (87%).
Respiratory failure
Respiratory failure was observed in 6 (1.4%) patients. Of these four patients presented a TLVR >350 mL. In 4 (66.7%) cases, respiratory failure developed during hospitalization (mean time 9.3±5.4 hours) and in the remaining 2 (33.3%) cases 4.2±1.7 months later. The treatment required invasive ventilation more than 24 hours in 3 (50.0%) cases, medical therapy in 2 (33.3%) cases and medical therapy with valve removal in 1 (16.7%) case. In all cases, respiratory failure resolved.
Pneumonia
Pneumonia was observed in 7 (1.7%) patients 7.5±2.3 months after the treatment. Six (85.7%) patients presented a TLVR >350 mL. Pneumonia resolved with medical therapy in 5 (71.4%) cases. In 2 (28.6%) patients the valves were removed and in only one they were re-implanted obtaining a TLVR >350 mL.
COPD exacerbation
COPD exacerbation was observed in 4 (0.9%) patients. Only one patient presented a TLVR >350 mL. In one case COPD exacerbation occurred during hospitalization and its treatment required the removal of the valve. In the other three cases it occurred 2.9±0.7 months after treatment and resolved with medical therapy alone.
Migration and/or expectoration
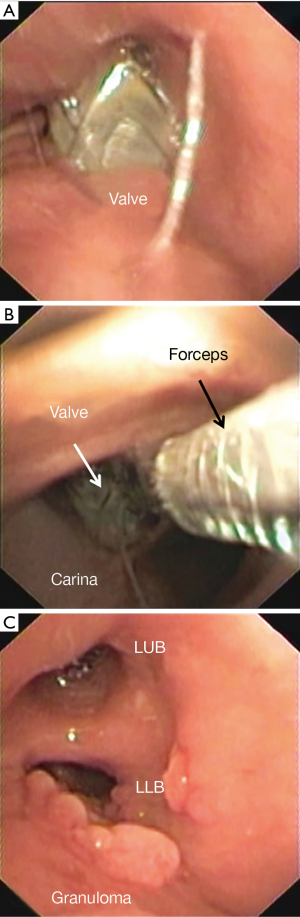
Migration and/or expectoration were observed in 9 (2.1%) of patients (Figure 4). In 3 (33.3%) patients it occurred during hospitalization (mean time: 32±11 hours from implant) and in 6 (66.7%) of patients 3.7±2.1 months from implant. No patient had a significant TLVR. In all cases the valves were re-implanted after a mean time of 3.5±1.3 months obtaining a TLVR >350 mL in six patients. An example of valve migration is reported in Figure 4.
Hemoptysis
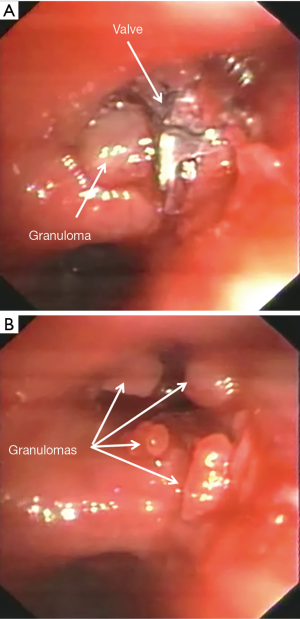
Hemoptysis was observed in 8 (1.9%) patients 10.0±6.9 months after valve implant. In one case hemoptysis was massive due the presence of a micetoma and required embolization. In five cases it was mild and due to granuloma formation (Figure 5). In all cases the valves were removed and in 2 of these the granulomas were resected with laser. In the remaining two patients, hemoptysis resolved with medical therapy. Only 1 (20.0%) patient underwent re-implantation of valve obtaining a TLVR >350 mL. An example of hemoptysis due to granuloma is reported in Figure 5.
Clinical outcome
Data are summarized in Table 3. The rate of patients with TLVR >350 mL did not change after the treatment of complications (61.6% vs. 59.8%, P=0.9) confirming that whatever complication did not affect the lobar atelectasis rate.

Full table
Patients with TLVR >350 mL (n=64) compared to those with TLVR <350 mL (n=43) had a significant improvement in delta FEV1 (19.0%±3.9% vs. 3.0%±0.9%; P=0.0003); in delta RV (−10.0%±4.8% vs. −4.0%±2.9%; P=0.002); in delta 6MWT (33.0±19.0 vs. 12.0±6.3 metres; P=0.001); and in delta SGRQ (−15.0±2.9 vs. −8.0±3.5 points; P=0.01). Only patients having TLVR ≥350 mL met or exceeded the MCID cut-off criteria for FEV1 (19.0%±3.9%), RV (−10.0%±4.8%), 6MWT (33.0±19.0 metres), and SGQR (−15±2.9 points) while in patients with TLVR <350 we observed only the MCID for SGQR (−8.0±3.5 points).
Discussion
EBV treatment in patients with heterogeneous emphysema and with proven absence of CV provided significant clinical benefits (2-8). The lobar atelectasis obtained with EBV implant mimics the same physiologic effects of LVRS in terms of reduction of RV and improves respiratory mechanics a larger application and shorter length of hospital stay, and reduced morbidity and mortality. EBV treatment is per se a safe and well tolerated procedure. A recent metanalysis by Liu et al. (14) showed that EBV treatment in comparison with standard medication and sham EBV did not increase the overall rate of mortality and morbidity except for mild hemoptysis which was more common in the EBV groups than in control groups (P=0.03). However, EBV treatment as well as most thoracic procedures can be accompanied by severe and less severe complications as reported in randomized clinical trials (RCTs) (2-7). Despite all, this issue is not largely evaluated in the literature. All published papers (15-17) focused on the incidence, treatment and clinical outcome of pneumothorax which is the most frequent EBV complication but there are other potential complications related to EBV treatment that remain under evaluated. To overcome these limits, we conducted this retrospective multicenter study with the aim of evaluating all complications due to EBV treatment and their impact on clinical outcome, an issue which has not been reported before.
As expected, pneumothorax was the most frequent complications (17.3% of patients). Early trials as the US VENT (2) and its European cohort (3) reported a lower rate of pneumothorax (4% and 8%, respectively) than the more recent (3,4,6) where it ranged between 17% and 23%. The reason for this different pneumothorax is the better selection of patient with only CV-negative being treated. In the VENT study (2) 67% of patients in the treatment group presented incomplete fissure at CT scan (CV-positive patients) while more recent trials as STELVIO (3), LIVE (4), and TRANSFORM (6) enrolled only patients defined as CV-negative (complete fissure at visual CT scan and/or CV-negative at Chartis). Since pneumothorax seems to be an indicator of successful valve therapy, there is the tendency among physicians to consider it as a “good complication” (if it is ethically right to consider a complication as “good”). In line with this evidence, in our series the incidence of pneumothorax was higher in responders (having a TLVR more than 350 mL) compared to non-responders. Despite all, 10/73 (13.7%) patients without significant lobar collapse experienced pneumothorax. This phenomenon is also reported in the European cohort of the VENT study (3) and in the study of Gompelmann et al. (15) where 7/25 (28%) and 42/70 (60%) patients, respectively, developed pneumothorax despite no lobar collapse. In agreement with previous RCTs (3,4) and Gompelmann’s study (15), pneumothorax (86%) occurred within the first 72 hours confirming need to prolong hospitalization for a minimum of 72 hours. In 3 (4.1%) of our patients, pneumothorax occurred during the procedure. Thus, a sudden and an unexpected respiratory failure during or immediately after valve implantation should make you suspect a pneumothorax and physicians should be ready for an emergency treatment (i.e., chest drainage). In theory, the rapid re-expansion of the ipsilateral untreated lung due to sudden atelectasis favors the rupture of parenchyma with consequent pneumothorax. On the other hand, 10/73 (13.7%) of our patients developed pneumothorax several months after treatment making difficult to define whether this event is due or not to the valve treatment.
Due to the retrospective nature of the study, each participant centers managed pneumothorax according to their experience, but in all cases the treatment was in line with standard recommendations (16,17). A “watch and wait strategy” was applied in 31.5% of cases for asymptomatic pneumothorax. Chest tube drainage insertion was required in 68.5% of cases for symptomatic pneumothorax. This datum is in line with the STELVIO study (3) and Gompelmann’s study (15) where 80% of patients with pneumothorax underwent chest insertion. In 16.4% of cases, persistent air-leaks (more than 5 days) required removal of the valve. The STELVIO study (3) and Gompelman’s study (15) reported a rate of valve removal of 50% and 44%, respectively, for the management of pneumothorax and persistent air-leaks. In all cases the persistent air leaks resolved with no need for surgery.
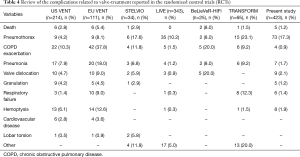
Other complications seen in our series were pneumonia (1.7%), COPD exacerbation (0.9%), respiratory failure (1.4%), valve migration or expectoration (2.1%) and hemoptysis (1.9%) caused by micetoma or granulation. Their rates were similar to RCTs studies summarized in Table 4 and resolved with appropriate medical treatment and in selected cases with valve removal (nine cases). Only COPD exacerbation presented a lower rate in our than in other series. In theory, COPD exacerbation is a phenomenon that occur in all COPD patients. Thus, in our study it could be under evaluated due to the difficult to define whether it was related or not to valve treatment considering the retrospective nature of the study and the lack of a control group.
Full table
EBV treatment is a reversible procedure and complete resolution of complications without further side effects was observed in all patients where the valves were removed (21 patients). However, we found that again the presence of lobar atelectasis after the resolution of the complication was the only predictive factor for significant clinical improvement. This is line with the VENT study and Gompelmann’s analysis. In the VENT study (2), patients who experienced pneumothorax had similar clinical benefits in terms of FEV1 and 6MWD compared to the subgroup of patients with complete fissure and without any complication. In Gompelmann’s series (15), 3 months after pneumothorax, valve therapy was associated with significant improvement in all lung function parameters except VC in patients with lobar atelectasis. Thus, patients, in whom the valves are removed for the management of complication, should be reviewed for re-implanting valves in order to obtain lobar collapse. Furthermore, we observed in 23/73 (31.5%) patients with pneumothorax the reexpansion of the target lobe despite the valves were not removed. In theory, the abnormal movement of the lung during the pneumothorax could favor a small dislocation of the valves, resulting in a lack of significant TLVR. Thus, in these patients a CT scan followed by bronchoscopy check is indicated in order to diagnose any valve dislocation.
Our study confirmed that delayed complications can occur also several months after valve implant, underlying the necessity of a prolonged follow-up. Because the patients will often back under the care of their primary physicians, it is mandatory to inform the patient and his family regarding the most common clinical signs of the complications. A sudden and unexpected chest pain associated with respiratory failure could be related to pneumothorax, a loss of clinical benefit or increased coughing could be associated with valve migration (18,19), hemoptysis with granuloma formation, while persistent infection with pneumonia. In this way, the patient and his/her family are able to understand when to alert their threatening physicians and/or refer to a local hospital for an emergency treatment. EBV treatment is not largely performed and in the most hospital there is a lack of knowledge about the role, the function, and the complications of valves. Thus, establishing a link with minor hospitals should be encouraged in order to allow the early transfer of the patient to the threatening expert centers after stabilization of his/her clinical condition. BLVR has been developed for people considered to be too disabled to withstand LVRS, thus the treatment of any complication could be a challenge due to poor clinical condition of the patient. There are cases of death (20) reported in patients who had pneumothorax after discharge from the hospital. In the BeLieVeR-HIFi (6) study two patients died and one occurred as a complication of valve removal. Thus, BLVR should be performed in expert centers by a multi-disciplinary approach including thoracic surgeons, anesthesiologists and pulmonologists.
The retrospective and multicentric nature of the study, the different experience among participating centers, and the non-standardized protocol for the treatment of complications are all factors that should be considered before drawing definitive conclusions from our results.
Conclusions
Despite safe and well tolerated, EBV treatment could be associated with early and delayed complications. However, it is a reversible procedure and complete resolution of complications is obtained with or without valve removal. Complications did not have significant impact on clinical outcome in patients with lobar atelectasis. However, it is crucial to remember that some of these complications could be potentially life-threatening if not promptly treated.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study design was approved by the Ethic Local Committee of the two Coordinating Centers (University of Campania “Luigi Vanvitelli” and Sapienza University-Rome Sant’ Andrea Hospital). Patients signed a written informed consent for the EBV treatment and they were aware that their data could be anonymously analyzed for scientific purposes only.
References
- Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. [Crossref] [PubMed]
- Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233-44. [Crossref] [PubMed]
- Herth FJ, Noppen M, Valipour A, et al. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J 2012;39:1334-42. [Crossref] [PubMed]
- Klooster K, ten Hacken NH, Hartman JE, et al. Endobronchial Valves for Emphysema without Interlobar Collateral Ventilation. N Engl J Med 2015;373:2325-35. [Crossref] [PubMed]
- Skowasch D, Fertl A, Schwick B, et al. A Long-Term Follow-Up Investigation of Endobronchial Valves in Emphysema (the LIVE Study): Study Protocol and Six-Month Interim Analysis Results of a Prospective Five-Year Observational Study. Respiration 2016;92:118-26. [Crossref] [PubMed]
- Davey C, Zoumot Z, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet 2015;386:1066-73. [Crossref] [PubMed]
- Kemp SV, Slebos DJ, Kirk A, et al. TRANSFORM Study Team. A Multicenter Randomized Controlled Trial of Zephyr Endobronchial Valve Treatment in Heterogeneous Emphysema (TRANSFORM). Am J Respir Crit Care Med 2017;196:1535-43. [Crossref] [PubMed]
- Fiorelli A, D'Andrilli A, Anile M, et al. Sequential Bilateral Bronchoscopic Lung Volume Reduction with One-Way Valves for Heterogeneous Emphysema. Ann Thorac Surg 2016;102:287-94. [Crossref] [PubMed]
- Fiorelli A, Petrillo M, Vicidomini G, et al. Quantitative assessment of emphysematous parenchima using multidetector-row computed tomography in patients scheduled for endobronchial treatment with one-way valves†. Interact Cardiovasc Thorac Surg 2014;19:246-55. [Crossref] [PubMed]
- D'Andrilli A, Vismara L, Rolla M, et al. Computed tomography with volume rendering for the evaluation of parenchymal hyperinflation after bronchoscopic lung volume reduction. Eur J Cardiothorac Surg 2009;35:403-7. [Crossref] [PubMed]
- Gompelmann D, Eberhardt R, Slebos DJ, et al. Diagnostic performance comparison of the Chartis System and high-resolution computerized tomography fissure analysis for planning endoscopic lung volume reduction. Respirology 2014;19:524-30. [Crossref] [PubMed]
- Fiorelli A, Santini M, Shah P. When can computed tomography-fissure analysis replace Chartis collateral ventilation assessment in the prediction of patients with emphysema who might benefit from endobronchial valve therapy? Interact Cardiovasc Thorac Surg 2018;26:313-8. [Crossref] [PubMed]
- Koster TD, Slebos DJ. The fissure: interlobar collateral ventilation and implications for endoscopic therapy in emphysema. Int J Chron Obstruct Pulmon Dis 2016;11:765-73. [Crossref] [PubMed]
- Liu H, Xu M, Xie Y, et al. Efficacy and safety of endobronchial valves for advanced emphysema: a meta-analysis. J Thorac Dis 2015;7:320-8. [PubMed]
- Gompelmann D, Benjamin N, Kontogianni K, et al. Clinical and radiological outcome following pneumothorax after endoscopic lung volume reduction with valves. Int J Chron Obstruct Pulmon Dis 2016;11:3093-9. [Crossref] [PubMed]
- Valipour A, Slebos DJ, de Oliveira HG, et al. Expert statement: pneumothorax associated with endoscopic valve therapy for emphysema--potential mechanisms, treatment algorithm, and case examples. Respiration 2014;87:513-21. [Crossref] [PubMed]
- Slebos DJ, Shah PL, Herth FJ, et al. Endobronchial Valves for Endoscopic Lung Volume Reduction: Best Practice Recommendations from Expert Panel on Endoscopic Lung Volume Reduction. Respiration 2017;93:138-50. [Crossref] [PubMed]
- Fiorelli A, Reginelli A, Santini M. Endobronchial Valve Migration: A "WhistleBlower". J Bronchology Interv Pulmonol 2016;23:e24-6. [Crossref] [PubMed]
- Fernandez-Bussy S, Labarca G, de Oliveira H. Endobronchial Valve Migration Secondary to Changes in Bronchial Diameter After the Initial Treatment. J Bronchology Interv Pulmonol 2018;25:e7-9. [Crossref] [PubMed]
- Caviedes IR, Labarca G, de Oliveira HG, et al. Non-Answered Questions in Patients with Endobronchial Valve Placement for Lung Volume Reduction. Respiration 2018;95:269-72. [Crossref] [PubMed]