Antegrade cardioplegia as a possible cause of acute saphenous vein endothelial damage in patients undergoing on pump coronary artery bypass surgery
Introduction
Since the introduction of saphenous vein grafting (SVG) in the late 1960s (1,2), coronary artery bypass grafting (CABG), with or without arterial conduits, remains the standard treatment for the management of intractable angina due to coronary artery occlusive disease. Despite the superiority of arterial graft patency over that of vein grafts, the multivessel nature of coronary artery disease and ready availability of saphenous vein still result in its use in over 70% of CABG procedures (3). However, it became evident later that the saphenous vein provided only palliation of the ongoing process, which is further complicated by vein graft atherosclerosis (4-6).
Approximately the 15% to 20% of vein grafts occlude in the first year, and half of them occlude within the first 2 weeks (5,7). Early graft occlusion appears to result from graft thrombosis (8). Thereafter, the annual occlusion rate is 1% to 2% from 1 and 6 years and 4% to 5% from 6 to 10 years. At 10 years, approximately 60% of vein grafts are patent; only 50% of these vein grafts remain free of significant stenosis (6,9,10). The pathology in the vein grafts documented by angiography and histologic examinations includes acute thrombosis and intimal hyperplasia during the first postoperative year and onset of progressive atherosclerosis beyond 3 to 5 years (6,9,11,12). The terminology of the atherosclerotic vein graft process is called venous graft disease (13-16). Current attempts to limit SVG stenosis and subsequent occlusion include surgical (technical) considerations, lipid-lowering and antiplatelet medications (16).
Literature data shows that there are many attempts to explain the factors that lead to SVG atherosclerotic disease. Some studies mention: (I) the endothelial damage caused during graft harvesting whereas others attribute it (II) to activated neutrophile adherence to the vein endothelium or even molecular factors, such as (III) a possible inhibition in nitric oxide (NO) production of the graft inner layer. Other technical factors involved include (IV) hydrostatic distention of the vein, (V) delay of implantation, (VI) low vein graft blood flow, (VII) composition of solutions used to store the vein graft and (VIII) composition of solutions used to perfuse the vein. All such factors are proven correct in several studies.
Unfortunately, these interventions fail to address the problem satisfactorily and venous graft disease still remains a persistent problem, leading to recurrent angina and a 10% to 15% incidence of redo CABG (13,14,16).
Previous studies have discussed on the possibility of harmful effect of either blood or crystalloid cardioplegia on the vein graft endothelium. However, these studies were more focused on the reduction of contractility of the endothelium and its function in general, as a result of loss of vascular supply of the vein grafts and denervation. Vein graft disease differs from arterial atherosclerosis in that its natural history is much shorter and the date of onset is clearly defined (i.e., graft implantation). This process is therefore potentially amenable to strategies that may inhibit its progression.
This study aims at examining the potassium cardioplegic solution as a cause of acute vein endothelial damage possibly leading to late vein graft atherosclerosis. Specifically, the damage of the vein graft endothelium after deliverance delivery of cardioplegic solution through its lumen was evaluated and differences were studied.
Methods
Study population
A sample of 52 patients, 36 males and 16 females, aged 44–81 years (mean age 68±8.5 years) who had undergone on pump CABG with at least one (1) vein graft, were studied. The participants were recruited from our department of Cardiac Surgery and from 2012 to 2014. All patients had coronary disease and required surgical revascularization. A total of 600 candidates were screened and the exclusion criteria included patients with a concomitant procedure during the primary operation, diabetics on insulin and smokers. No significant differences with regard to demographics, preoperative risk factors, or medication use were noted between patients. Intraoperatively collected data such as ejection fraction, conduit diameter, target size and quality, and inotrope requirements were also similar.
The study had a prospective study design based on the research hypothesis and a specifically designed protocol as it will be subsequently presented. All the candidate participants were asked to participate and all of them provided with informed consent. The study was approved by the ethics committee and the scientific board of our Institution.
Surgery
Four surgeons experienced in on pump CABG enrolled the patients. Internal mammary conduits were used in all patients. The greater saphenous vein was harvested from each patient using the standard open approach. After harvesting and careful double ligation of the branches, veins were mildly dilated in a controlled manner with use of heparinized blood of the patient and then stored in it (16). A segment of the prepared saphenous vein was cut off and subsequently stored for histological analysis.
The surgical procedure progressed as usual. The patients’ temperature was allowed to drift to 32 °C. The induction cardioplegic solution contained 1,000 mL Ringer’s lactated, 2 vials of St. Thomas solution, 40 mmol sodium bicarbonate and 67 mmol potassium all mixed with blood at a 1:4 ratios.
Immediately after each vein anastomosis to the targeted coronary artery, the vein was cut at the appropriate length. Antegrade maintenance cardioplegia was then delivered directly to the aortic root and at the same time through the vein graft towards the targeted coronary artery. The maintenance cardioplegic solution contained 1,000 mL Ringer’s lactated, 2 vials of St Thomas solution, 40 mmol sodium bicarbonate and 40 mmol potassium all mixed with blood at a 1:4 ratios. The temperature of the solution was 6–8 °C. The infusion rate was kept between 150 and 200 mL/min. In that way the graft was not over distended. The amount was 300 mL for each delivery. The rest of the operation was conducted in the usual manner.
Saphenous vein specimens’ preparation
After being removed from the leg, a vein cannula was placed in the distal end of the vein and secured into position with a silk ligature. Heparinized blood was injected by hand using a 60 mL pressure controlling syringe (Vasoshield-Maquet®) to localize unsecured branches (17). The pressure controlling syringe helps protect vessels from over distension and potential endothelial damage during standard surgical preparation steps. It works by limiting the internal “flushing” pressure when harvested grafts are irrigated in preparation for use in CABG surgery (18-21). The full length of the vein was then distended to relieve spasm and therefore all parts were exposed to the same distending pressure. At that point, the last segment of the vein was cut off and stored for histological analysis (group A). The vein was then divided into segments equal to the number of the anastomoses required. The vein segments were then stored in heparinized blood at room temperature until the surgeon was ready to sew each vein graft.
Immediately after the performance of the last vein graft anastomosis to the aortic root, the excess proximal SVG length of the last vein segment (including the vein cannula) was cut off and stored for histological analysis (group B). Thus, the stored segment had only been exposed to antegrade cardioplegia once.
Endothelial assessment and histological analysis
The endothelium of the two segments was examined histologically. Endothelial cells (ECs) comprise the single cell-thick, continuous lining of the entire cardiovascular system, collectively called the endothelium. ECs uniquely contain Weibel-Palade bodies, 0.1 µm-wide, 3µm-long membrane-bound storage organelles, that contain von Willebrand factor (vWF). These cells can be identified immunohistochemically with antibodies to PECAM-1 (CD34, a protein localized to inter endothelial junctions) (22,23). Both nuclear and cytoplasmic staining was estimated as positive.
All vein graft specimens were formalin fixed and paraffin embedded at the Department of Pathology, of our hospital. The evaluation of the histological findings was performed blinded by two separate Pathologists according to the international criteria.
For light microscopy, the grafts were left in the 10% neutral buffered formalin, after their division into two groups (group A: without cardioplegic solution and group B: with cardioplegic solution run through them). The specimens were dehydrated in ascending alcohol concentrations and embedded in paraffin. Sections 4 µm in thickness were stained with Gill III Hematoxylin-Eosin method and examined with light microscope.
Immunohistochemistry (IHC)
Protein expression analysis was evaluated in routinely fixed and paraffin wax-embedded tissues. Sections of different specimen (4 μm thickness each) were applied to Dako Real™ Capillary Gap Microscope Slides and were left to incubate overnight at 55 °C. The sections were then deparaffinized by two consecutive treatments with xylene for 5 minutes each. Rehydration was performed with graded ethanol (90%, 80%, and 50%) for 4 minutes each. Antigen retrieval was achieved by boiling the sections in a steamer with 20× target retrieval solution (DAKO) for 45 minutes. Immunostaining reactions were performed in an automated immunostaining system (Tech-Mate™ 500 Plus, DAKO). In the present study, the following primary antibody was used: monoclonal mouse anti-human CD34 Class II, Clone QBEnd10 (DAKO) diluted 1/20 (v/v). Primary antibodies dilutions were performed in REAL™ Antibody Diluent (DAKO). The immunostaining reaction was developed using REAL™ EnVision™ Detection System, Peroxidase/DAB+, Rabbit/Mouse (DAKO).
In each section, the percentage of total vessel circumference that stained positive for CD34 was calculated using image analysis software (REAL™ EnVision™ Detection System). Washing reagents were used from the ChemMate™ Buffer Kit (DAKO). Sections with normal vessel ECs were used as a positive control. A section that did not receive any primary antibody was used as a negative control. Two independent, masked reviewers assessed the endothelial integrity of each sample of every segment. The final average endothelial integrity each reviewer arrived at for the segment as a whole was then taken, and these two values were averaged. The variability between the two reviewer’s scores was less than 10%.
Statistical analysis
The primary endpoint of this study was the acute endothelial damage caused by antegrade cardioplegia when delivered through saphenous vein conduits. In prior studies, an incidence of early (in the first 2 weeks post CABG) graft failure of 6.5% has been reported (5,7,24). Assuming a strong effect of conduit endothelial damage on early thrombosis, power analysis indicated that 50 patients were sufficient to demonstrate a relationship at P=0.05 and power confidence of 0%.
The normality of the venous specimens’ characteristics (i.e., length and endothelial damage) as continuous variables was tested graphically according to histograms, P-P and Q-Q plots. The graphs indicated that all the characteristics tended not to follow the normal distribution. Thus, the results are presented as median (1st and 3rd quartile). Furthermore, the non-parametric Wilcoxon test was applied for comparisons of venous specimens’ characteristics with and without the antegrade cardioplegia for both genders, considering the skewedness of the data and the sample size (men n=36 and women n=16). Two-sided P<0.05 was considered statistically significant. STATA version 9 software was used for all calculations (STATA Corp., College Station, TX, USA).
Results
The analysis of data on the endothelial damage is illustrated in Figure 1. The endothelial damage of vein specimens appeared to be increased significantly with exposure to antegrade cardioplegia in male and female patients (P from Wilcoxon tests <0.001, for both genders). In fact, the median endothelial damage without exposure to antegrade cardioplegia was 25% (range, 20–30%) for men and 30% (range, 20–34%) for women. The correspondent values of endothelial vein graft damage when the graft was exposed to antegrade cardioplegia were 65% (range, 60–75%) and 70% (range, 65–75%).

The results from the analysis of data on the length of vein specimens are presented in Figure 2. The median length of vein specimens without the antegrade cardioplegia was 3.75 cm (range, 1.5–5 cm) for men and 4 cm (range, 3–4.5 cm) for women. The correspondent values with the antegrade cardioplegia were 7 cm (range, 3–8 cm) and 6 cm (range, 3–7 cm). The comparisons of the length of vein specimens with and without the antegrade cardioplegia showed statistically significant differences in both genders (P from Wilcoxon test <0.001 for men and P=0.001 for women).
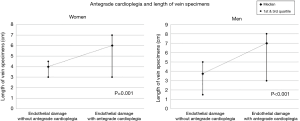
The increase in the length and endothelial damage of vein specimens with the antegrade cardioplegia was also significant in both age groups of 40–65 years (n=16) and >65 years (n=36) (P from Wilcoxon tests <0.001, for both). This finding may be of use in future studies. It seems that the length of the vein specimens which were send for histological analysis directly correlated with the amount of the endothelial damage. Specimens with length of at least 3 cm allowed for more sections available for microscopy and IHC. This possibly had a positive effect on the accuracy of the estimation of the endothelial damage.
Discussion
In the present study, endothelial damage of the vein graft endothelium is directly related to exposure of the endothelium to maintenance cardioplegia solution. This happens when cardioplegia is administered in the antegrade fashion through vein grafts anastomosed to the native coronary arteries (CABG procedure). Endothelial damage is proven to be substantial and can only be attributed to the antegrade cardioplegia deliverance. Although several investigations have helped clarify the role that neointimal hyperplasia plays on the long-term outcome of veins placed in the arterial circulation (25,26), little attention has been given to the pathophysiologic importance of damage sustained by the conduit during deliverance of antegrade cardioplegia through it (27). Although it seems eminently logical, no prior prospective study has established a direct link between the antegrade maintenance cardioplegic solution and the acute damage of the endothelium of the vein graft. This may possibly lead to early or late vein graft failure.
The vascular endothelium is a hemocompatible monolayer of mesenchymal cells and forms a barrier between the circulating blood and extravascular space (24,25). The preservation of EC viability is vital for inhibiting early pathologic changes and the long-term patency of vascular grafts (19,28-32). Saphenous vein was introduced as a bypass graft in the late 1960s and atherosclerotic problems were soon recognized. Restenosis of venous bypass grafts can occur as a healing and remodeling response to the initial tissue injury in a poorly regulated process. Pathologic SVG proliferation and associated intimal hyperplasia is a critical early factor which most probably leads to graft atherosclerosis and subsequent failure (19,31,32). Compared with atherosclerosis in the arteries that begins in childhood and develops slowly, the venous graft disease is a rapidly progressing condition which affects thousands of patients receiving CABG operation each year worldwide. Given the fact that the 10-year patency of the IMA graft is nowadays established, venous graft disease seems to be one of the most important causes that lead to CABG reoperations.
Venous graft lesions are generally categorized as acute and chronic. Chronic lesions consist mostly of local thickening of the native vessel wall or atherosclerotic plaques on the endothelium. For these lesions little can be done in conventional CABG surgery since the native greater saphenous vein continues to be the graft of choice in such operations. Acute vein graft disease is most commonly caused by local or extensive destruction of the endothelial layer and edema of the sub endothelial layer. Other causes seem to be ischemia in the vessel wall and necrosis of muscle cells in the sub endothelial layer and thrombus adhesion in the vessel lumen (25-33).
While surgical technique has received the majority of the blame for saphenous vein graft failure rates, which are reported to approach 10% in the first postoperative month (5,7,24), very little literature exists examining other factors that may influence success of the graft. Most authors agree that acute vein graft injury inadvertently leads to atherosclerotic disease in the future. It also seems that most efforts are focused in ways to prevent or treat this disease.
In this study, antegrade maintenance cardioplegic solution is assessed as a possible cause of endothelial vein damage. This kind of damage can possibly lead to necrosis, neutrophile infiltration, platelet adhesion and accumulation and subsequent formation of atheromatous plaques (19,25-33). The administration of antegrade cardioplegia through vein grafts at the completion of each distal anastomosis is a common practice. It is considered a substantial aid towards better myocardial protection, especially in cases of near or totally occluded arteries or in cases of severe left main stenosis, mainly for anatomical reasons. Apart from the aforementioned indications, many cardiac surgeons use routinely the vein grafts to deliver cardioplegia in their day-to-day practice through millilumen antegrade lines.
The cardioplegic solution is in many circumstances of low temperature. In our series, the temperature varied between 6 and 8 °C. Moreover, it is rich in potassium, not only because it contains 40 mmol of potassium but also because of the potassium contained in the St Thomas solution and the potassium contained in the blood which is mixed with the cardioplegic solution. The combined effect of these two factors, low temperature and potassium may affect the vein endothelium and thus play an important role for initiation of the acute endothelial injury process.
In the literature many reasons have been described so far as possible causes of the endothelial injury of the vein grafts even before they are anastomosed and placed on the arterial side of the circulation. The vein specimens who were not exposed to antegrade cardioplegia, (group A) present indeed a mild albeit significant endothelial damage (Figure 3). There are many studies (12,17-19,30,34-36) that attempt to explain the mild endothelial damage of the procured vein graft. Recent studies (14,15) imply that SVG harvested with minimal handling, using a no-touch technique such as endoscopic vein harvesting have minimal endothelial damage. This needs to be studied further.
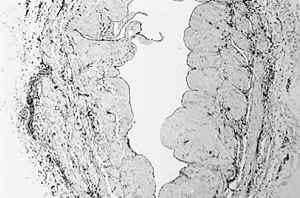
Vein distention during preparation might also play a vital role in vein damage in group A. Moderate pressure distention can cause reduction of the vascular endothelium up to 10%. Excessive distention causes separation of ECs, disruption of medial smooth muscle cells and stretches the intima (18-21). Therefore, there was an effort in this study, as described at the surgical technique section, to avoid extensive distention which could increase the affected areas and mask the direct effect of potassium rich cold cardioplegia to vein endothelium.
Apart from the aforementioned ways of acute vein damage, many SVG grafts might present chronic lesions which were present already at the time of harvesting. These lesions are mainly attributed to predisposing factors, such as smoking, varicosity, diabetes mellitus, and hyperlipidaemia (16,35). Therefore, active smokers, diabetics on insulin, and those with varicosities were excluded from the study population. However, age is not considered as a predisposing factor for chronic vein graft lesions (32). Thus, patients’ age was not an exclusive criterion in this study.
Vein grafts with direct exposure to antegrade cardioplegia (group B) present with excessive damage to the endothelial layer (Figure 4). This is proved with use of CD34, which is a sensitive immunohistochemical index for vessel endothelia (23). The tissue samples were examined under light microscopy and the endothelial damage was measured using a hemi quantitative method. The results prove the excessive damage to the SVG endothelium which is caused when maintenance cardioplegia solution mixed with blood at 1:4 ratios run through them. These acute endothelial lesions lead to cell deficiency and damage to the vein cytoskeleton (19,27,32,34). The exact mechanism needs to be studied further. There might be an increase in the genetic expression of adhesion molecules, growth factors and the production of proteins. Moreover, as cardioplegia runs through the veins, it washes damaged cells away which might increase vasoconstriction and further impairment of the vein graft.
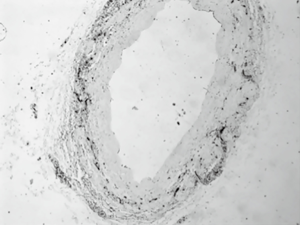
The damaged endothelial areas will most probably be covered by regenerated endothelium, usually after 1 to 2 weeks. However, since these areas are highly thrombogenic, platelets may have already begun to accumulate on them by that time. Therefore, the neoendothelium is forced to be laid over a carpet of platelets and fibrin already deposited. Later, this thrombotic material is replaced by proliferating smooth muscle cells which are considered a prime factor of vein graft atherosclerosis. Moreover, platelets release factors that enhance the development of intimal hyperplasia which in advance leads to SVG atherosclerosis (36,37).
The above-mentioned factors, combined with some more, such as a reduction in endothelial NO synthesis and release (16) and also the ischemia of the harvested vein due to loss of its vascular supply bed might explain the results of endothelial damage in vein graft segments not exposed to antegrade cardioplegia. On the other hand, the much more significant results of group B which consisted of vein graft segments with direct exposure to antegrade cardioplegia might be attributed to the maintenance cardioplegic solution.
The maintenance cardioplegic solution mixed with blood at 1:4 ratios is commonly used in myocardial protection strategies in on pump CABG. However, it is generally cold and potassium rich. As it runs through vein grafts, possibly causes acute endothelial damage to the SVG. This injury might be a predisposing factor to subsequent vein graft atherosclerotic disease. Further studies need to be undertaken though. The endothelial damage caused by the cardioplegic solution should be estimated in a more quantitative way. Moreover, the results indicate that there may be a need for alterations in the composition and temperature of the cardioplegic solution.
This study has several limitations. First, endothelial integrity and damage of the vein graft endothelium has been assessed in a qualitative way. Quantitative methods to detect endothelial disruption in the graft may improve the clinical relevance of our findings. Furthermore, our choice of vein graft storage solution (heparinized blood) may have played an additional role on endothelial integrity. Specialized storage solutions, similar to the one we used to store the excess vein segments, have been shown to improve endothelial integrity (38,39). Finally, the endothelial damage has been attributed to the maintenance cardioplegic solution in general and not to one or more specific substances of it or to its temperature. Further studies need to be undertaken to clarify whether specific substances of the cardioplegic solution or its temperature are the cause of the endothelial damage of the vein grafts.
Conclusions
In conclusion, the protective effect of antegrade cardioplegia through vein grafts to the arrested myocardium is well known. The temperature of the cardioplegic solution, however, needs to be studied further. The composition of that solution might also be in question. The potassium is potentially harmful to the vein inner layer as this study suggests. Protective substances might need to be added and harmful ones regulated so that antegrade cardioplegia will continue to protect the ischemic heart but also avoid damage to the endothelium of the vein grafts.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethics committee and the scientific board of our institution (IRB number: 223) and written informed consent was obtained from all patients.
References
- Garrett HE, Dennis EW, DeBakey ME. Aortocoronary bypass with saphenous vein graft. Seven-year follow-up. JAMA 1973;223:792-4. [Crossref] [PubMed]
- Favaloro RG. Saphenous vein graft in the surgical treatment of coronary artery disease: operative technique. J Thorac Cardiovasc Surg 1969;58:178-85. [PubMed]
- Izzat MB, West RR, Bryan AJ, et al. Coronary artery bypass surgery: Current practise in the United Kingdom. Br Heart J 1994;71:382-5. [Crossref] [PubMed]
- Angelini GD, Jeremy JY. Towards the treatment of vein bypass graft failure – a perspective of the Bristol Heart Institute. Biorheology 2002;39:491-9. [PubMed]
- FitzGibbon GM, Leach AJ, Keon WJ, et al. Coronary bypass graft fate. Angiographic study of 1,179 veins early, one year, and five years after operation. J Thorac Cardiovasc Surg 1986;91:773-8. [PubMed]
- Fitzgibbon GM, Leach AJ, Keon WJ, et al. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol 1996;28:616-26. [Crossref] [PubMed]
- Tatoulis J, Buxton BF, Fuller JA. Patencies of 2127 arterial to coronary conduits over 15 years. Ann Thorac Surg 2004;77:93-101. [Crossref] [PubMed]
- De Caterina R, Weksler BB, Alonso DR, et al. Functional endothelial damage by high-potassium cardioplegic solutions to saphenous vein bypass grafts. Surgery 1985;98:465-71. [PubMed]
- Bourassa MG. Fate of venous grafts: the past, the present, and the future. J Am Coll Cardiol 1991;17:1081-3. [Crossref] [PubMed]
- Campeau L, Enjalbert M, Leasperance J, et al. The relation of risk factors to the development of atherosclerosis in saphenous vein bypass grafts and the progression of disease in native circulation: a study 10 years after aortocoronary bypass surgery. N Engl J Med 1984;311:1329-32. [Crossref] [PubMed]
- Shuhaiber JH, Evans AN, Massad MG, et al. Mechanisms and future directions for preventions of vein graft failure in coronary bypass surgery. Eur J Cardiothorac Surg 2002;22:387-96. [Crossref] [PubMed]
- Shah PJ, Gordon I, Fuller J, et al. Factors affecting saphenous vein graft patency: clinical and angiographic study in 1402 symptomatic patients operated on between 1977 and 1999. J Thorac Cardiovasc Surg 2003;126:1972-7. [Crossref] [PubMed]
- Hilker M, Tellmann G, Buerke M, et al. Proliferative activity in stenotic human aortocoronary bypass grafts. Cardiovasc Pathol 2002;11:284-90. [Crossref] [PubMed]
- Schlitt A, Pruefer D, Buerke U, et al. Neutrophil adherence to activated saphenous vein and mammary endothelium after graft preparation. Ann Thorac Surg 2006;81:1262-8. [Crossref] [PubMed]
- Dashwood MR, Savage K, Dooley A, et al. Effect of vein graft harvesting on endothelial nitric oxide synthase and nitric oxide production. Ann Thorac Surg 2005;80:939-44. [Crossref] [PubMed]
- Goldman S, Zadina K, Moritz T, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol 2004;44:2149-56. [Crossref] [PubMed]
- Kurusz M, Christman EW, et al. Use of cold cardioplegic solution for vein graft distention and preservation: a light and scanning electron microscopic study. Ann Thorac Surg 1981;32:68-74. [Crossref] [PubMed]
- Manchio JV, Gu J, Romar L, et al. Disruption of graft endothelium correlates with early failure after off-pump coronary artery bypass surgery. Ann Thorac Surg 2005;79:1991-8. [Crossref] [PubMed]
- Thatte HS, Khuri SF. The coronary artery bypass conduit: I. Intraoperative endothelial injury and its implication on graft patency. Ann Thorac Surg 2001;72:S2245-52. [Crossref] [PubMed]
- Poston RS, Kwan MH, Gu J. Role of procurement-related injury in early saphenous vein graft failure after coronary artery bypass surgery. Future Cardiol 2006;2:503-12. [Crossref] [PubMed]
- Chong CF, Ong PJ, Moat N, et al. Effects of hydrostatic distention on in vitro vasoreactivity of radial artery conduits. J Thorac Cardiovasc Surg 2004;128:609-14. [Crossref] [PubMed]
- Fina L, Molgaard HV, Robertson D, et al. Expression of the CD34 gene in vascular endothelial cells. Blood 1990;75:2417. [PubMed]
- He XY, Antao VP, Basila D, et al. Isolation and molecular characterization of the human CD34 gene. Blood 1992;79:2296-302. [PubMed]
- Poston R, White C, Read K, et al. Virchow’s triad, but not use of an aortic connector device, predicts vein graft thrombosis after off-pump bypass. Heart Surg Forum 2004;7:E428-33. [Crossref] [PubMed]
- Zilla P, von Oppell U, Deutsch M. The endothelium: a key to the future. J Card Surg 1993;8:32-60. [Crossref] [PubMed]
- Lüscher TF, Tanner FC, Tschudi MR, et al. Endothelial dysfunction in coronary artery disease. Annu Rev Med 1993;44:395-418. [Crossref] [PubMed]
- Olinger GN, Boerboom LE, Bonchek LI, et al. Hyperkalemia in cardioplegic solutions causing increased cholesterol accumulation in vein grafts. J Thorac Cardiovasc Surg 1983;85:590-4. [PubMed]
- Davies MG, Hagen PO. Pathophysiology of vein graft failure: a review. Eur J Vasc Endovasc Surg 1995;9:7-18. [Crossref] [PubMed]
- Verrier ED, Boyle EM Jr. Endothelial cell injury in cardiovascular surgery: an overview. Ann Thorac Surg 1996;62:915-22. [Crossref] [PubMed]
- Sellke FW, Boyle EM Jr, Verrier ED. Endothelial cell injury in cardiovascular surgery: the pathophysiology of vasomotor dysfunction. Ann Thorac Surg 1996;62:1222-8. [Crossref] [PubMed]
- Mills NL, Everson CT. Vein graft failure. Curr Opin Cardiol 1995;10:562-8. [Crossref] [PubMed]
- Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation 1998;97:916-31. [Crossref] [PubMed]
- Lawrie GM, Lie JT, Morris GG. Vein graft patency and intimal proliferation after aortocoronary bypass: early and long term angiopathologic correlation. Am J Cardiol 1976;38:856-62. [Crossref] [PubMed]
- Murphy GJ, Angelini GD. Insights into the pathogenesis of vein graft disease: lessons from intravascular ultrasound. Cardiovasc Ultrasound 2004;2:8. [Crossref] [PubMed]
- Kouzi-Koliakos K, Kanellaki-Kyparissi M, Marinov G, et al. Morphological features and apoptosis in the left internal thoracic artery grafts before implantation. Int Angiol 2007;26:38-48. [PubMed]
- Borna C, Wang L, Gudbjartsson T, et al. Contractions in human coronary bypass vessels stimulated by extracellular nucleotides. Ann Thorac Surg 2003;76:50-7. [Crossref] [PubMed]
- Hata JA, Petrofski JA, Schroder JN, et al. Modulation of phosphatidylinositol 3-kinase signaling reduces intimal hyperplasia in aortocoronary saphenous vein grafts. J Thorac Cardiovasc Surg 2005;129:1405-13. [Crossref] [PubMed]
- Roubos N, Rosenfeldt FL, Richards SM, et al. FRACS improved preservation of saphenous vein grafts by the use of glyceryl trinitrate-verapamil solution during harvesting. Circulation 1995;92:II31-6. [Crossref] [PubMed]
- Thatte HS, Biswas KS, Najjar SF. Multi-photon microscopic evaluation of saphenous vein endothelium and its preservation with a new solution, GALA. Ann Thorac Surg 2003;75:1145-52; discussion 1152. [Crossref] [PubMed]


