Cyberknife® stereotactic radiation therapy for stage I lung cancer and pulmonary metastases: evaluation of local control at 24 months
Introduction
Stereotactic body radiotherapy (SBRT) is a curative option for patients with stage I NSCLC who refuse to undergo surgery or who are medically inoperable. Real-time tracking with CyberKnife® offers a highly precise treatment with narrow margins (1,2).
SBRT also appears to be a suitable alternative to surgery for lung metastases (3,4), although the literature is less abundant in this regard. Three-dimensional conformal radiotherapy (3DCRT) is used extensively to treat lung malignancies. Despite taking into account the target’s movement, loco-regional control and survival have proven to be disappointing with conventional radiation therapy even if the SPACE phase II randomized study showed encouraging results for high precision 3D radiotherapy. This is probably due to a reduced biological dose-effect and a lower conformity index (1).
Prior studies of CyberKnife® have reported local control rates of 90–97% at 2–3 years of follow-up, with low toxicities (1,5-7). Treatment with CyberKnife® can also allow for a sustainable local control rate of 89% with a median follow-up of 12 months in oligometastatic lesions, with few morbidities (8).
The main advantage of CyberKnife® stereotactic treatment is derived from the capacity for real-time monitoring of tumors. This can be achieved with Synchrony® after implantation of fiducial markers (e.g., gold markers or vascular coils) (9), or by monitoring the tumor itself with Xsight® Lung. This advantage is not available with Xsight® Spine, which primarily tracks the rachis.
Few studies to date have sought to evaluate the level of compliance or compared the rate of local control for different treatment techniques: gold seeds, coils, lung and spine.
The aim of our study was to identify factors influencing the probability of local control.
Methods
Tracking image-guided therapy techniques with CyberKnife®
Cyberknife® is a robotic radiotherapy system. A 6 MV photon beam linear accelerator is mounted on a high precision robotic manipulator. Millimetric accuracy of patient position is achieved thanks to the combination of the robotic system, a dual kV X-ray imaging system and a robotic couch where patient is lying (Figure 1).
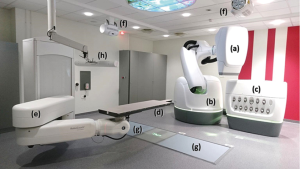
Three tracking methods car be used for thoracic lesions, two with respiratory movement compensation (Synchrony®, Xsight® Lung) and one without (Xsight® Spine).
Synchrony® technique
This method uses fiducial tracking, providing a level of accuracy less than 1.5 mm. It is based on the simultaneous identification of internal movements by localization of the tumor using fiducial markers, beforehand implanted near the tumor, external movements by an external camera-based tracking system for monitoring through the use of diodes, and a correlation model that evaluates the relationship between the internal and the external movements.
During treatment delivery, beam will record external diodes movements, predict tumor position using this correlation model and a correct beam position to compensate breath influence.
One fiducial is required to follow translations, while three or more are required to identify rotations.
The tracking error decreases significantly with more than three markers (e.g., 0.2 mm with three gold fiducial markers vs. 0.1 mm with five gold markers). Thus, implantation of four to six gold fiducial markers is recommended by the manufacturer. The recommended distance between the target and the fiducials implanted was according to (CyberKnife® technical training program), “fiducial placement principles” chapter and should not exceed 50 to 60 mm.
The recommended time frame for performing the scan is one week, so as to reduce errors related to movement of the fiducials between treatment planning CT scan acquisition and treatment. Treatment should begin as soon as possible after the CT-scan.
Gold fiducial markers are preferred but in case of severe respiratory failure, for several years use of endovascular markers (coils) can be made to eliminate the risk of pneumothorax. The procedure is performed by interventional radiologists under biplane angiography. This alternative has been proposed in the literature and appears to be similar to gold fiducial markers (8).
Xsight® lung tracking
This is based on identification of the tumor itself on X-Ray images. Algorithm searches the shadow of the tumor projected on each image. Tracking is the same as Synchrony® but only by replacing track of tumor shadow instead of fiducial markers.
The Xsight® Lung technique is an option to avoid fiducial implantation when the target is peripheral, exceeds 15 mm, and the imaging control during treatment does not overlap with the vertebrae: (Operating manual of CyberKnife®).
Xsight® spine tracking
In light of the high precision (<1 mm) of spinal tracking, the rachis is used as a marker. It is based on recording of a non-rigid frame that locates and tracks tumors close to spinal vertebrae without fiducials.
This method is provided to patients with tumors distant less than 50 mm from the middle of the posterior wall of the vertebra, when a non-invasive treatment is required. An ITV (internal target volume) is derived from a 4D scan to compensate uncertainty coming from the lack of breathing tracking during treatment.
Patients
From January 2013 to December 2013, details for all of the patients at our center who underwent stereotactic radiotherapy using CyberKnife® were retrieved from the hospital database. The eligibility criteria for this retrospective study were that the patients had to be at least 18 years of age and that they had a stage I lung cancer tumor (histological proof was not available for all patients) or lung metastases.
Primary lung tumors and metastases were treated by CyberKnife® radiation therapy without systemic treatment.
Gold seeds were implanted when pulmonary function and the patient’s health status were adequate to tolerate the risks associated with pneumothorax. Whenever possible, Xsight® Spine was preferred in case of poor pulmonary function, a poor health status, or refusal to be hospitalized. Coils were systematically provided for unfit patients who would be at significant risk in case of pneumothorax, and when the tumor was located more than 6 cm from the middle of the posterior wall of the vertebra.
Xsight® lung was chosen for tumors that were more than 20 mm in size, in order to avoid failure of tumor recognition, and when their location was favorable.
In order to compare practices and recommendations, the following characteristics of the treated tumors were collected: the location and size of the target, the total dose prescribed to the 80% isodose, the number of fractions, the number of gold fiducial markers or coils placed, the number of fiducials that were ultimately used, the distance of the gold markers or the coils relative to the target measured from the center of the GTV (gross tumor volume), and the distance of the GTV from the middle of the posterior wall of the vertebra for the Xsight®. The number of additional overnight hospitalization stays for complications after gold fiducial marker implantation was also recorded.
Treatment planning
Radiation therapy was performed at the same institute. The CT-scan used for treatment planning encompassed the entire lung, with millimeter-sized equidistant slices, and the acquisition included 10–15 cm above and below the tumor as well as the entire pulmonary volume. With the Xsight® Spine technique, we selected four representative phases of the respiratory cycle to achieve 4D CT-scanning.
The GTV was acquired from a 1 mm-sized slice CT scan, and it was performed in the pulmonary window. A 2 mm margin was considered for microscopic extension of tumor to define the CTV. The PTV was defined with a 3 to 5 mm margin around the CTV, generally 3 mm when markers were close to the PTV. For peripheral and central tumors, the prescribed doses were 60 Gy in 3 fractions (10) and 50 Gy in 4 or 5 fractions, respectively, delivered on the 80% isodose using a Ray-Tracing dose calculation algorithm (11).
Statistical methods
The numerical parameters are presented as medians and ranges; qualitative parameters are presented as frequencies and percentages.
Quantitative parameters were compared using the Mann-Whitney or the Kruskal-Wallis test and qualitative parameters with the Chi-square test or Fisher’s exact test. Some post-hoc tests were then performed with a Bonferroni correction.
Local control was described based on the Kaplan-Meier method from the date of the stereotactic radiotherapy CyberKnife® procedure. In case of relapse, the imaging date revealing the relapse was used. The cumulative probability of local control at 24 months was determined.
Bivariate Cox proportional hazard models were performed to compare local control according to clinical parameters. Results were expressed as hazard ratios and 95% confidence intervals.
Analyses were performed using SAS version 9.3 software (SAS Institute Inc., Cary, NC, USA).
The level of significance was set at 5%.
Results
Ninety-five patients were included in the study for a total of 100 lung tumors. Ninety patients had one tumor and five of them had two tumors. Of the 100 tumors, 35 were treated with gold seeds, 15 with coils, 7 with Xsight® Lung, and 43 with Xsight® Spine.
The theoretical minimum number of four fiducials was never observed in patients with gold seeds or coils. Fifteen patients were monitored with one implanted fiducial. There were 24 patients with two implanted fiducials, of whom only 9 had one that was monitored while for fifteen patients both of the fiducials were monitored. There were a further eleven patients with three implanted fiducials, of whom eight had two fiducials that were monitored and three patients for whom all three fiducials were monitored.
The required distance from the target to the fiducials (<60 mm) was observed for 97% (n=34) of the tumors treated with gold seeds and 67% (n=10) of the tumors treated with coils. The minimum of seven days between fiducial implementation and preparation for the scan was the case for 100% of the tumors treated with gold seeds.
For the Xsight® Lung technique, the minimum size required for detection of the target (15 mm) was observed for all of the patients. For the Xsight® Spine technique, the maximum distance of the target relative to the posterior wall of the vertebra (<50 mm) was observed for 42% (n=18) of the tumors.
Tumor characteristics
Of the 100 tumors, 71% were primary tumors and 29% were secondaries. Sixty-seven peripheral tumors received 60 Gy in 3 fractions; 28 central tumors received 50 Gy in 4 to 5 fractions; and, in case of reirradiation, five tumors received between 30 and 45 Gy in 3 to 5 fractions. The results for each technique are shown in Table 1. The dimension of the target was not significantly different according to the technique (P=0.173), although it appears to be somewhat higher for the Xsight® Lung procedure. The median distance of the target relative to the fiducials was 19 mm (95% CI, 0–70) for 62 gold seeds and 36 mm (95% CI, 0–132) for 31 coils. The distance was significantly less with the gold seeds compared to the coils (P<0.001).
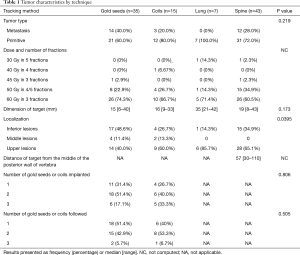
Full table
Out of the 35 patients with gold markers, 23 patients (66%) experienced complications due to intra-alveolar hemorrhaging or pneumothorax. This led to an increase in the number of additional overnight hospital stays for 26% of the patients: 11 patients were placed under observation for more than one night, while five patients underwent chest tube placement. None of the patients were hospitalized for complications following the installation of coils.
Local control
The results for local control are shown in Table 2.
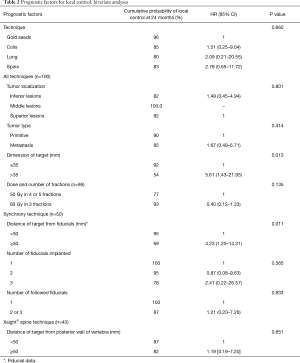
Full table
The median follow-up was 24 months, with an interquartile range from 12 to 28 months. The probability of local control (LC) at 24 months was 88% (95% CI, 78–94%).
The rate of LC did not differ according to the technique (P=0.548). The LC rate did not differ based on the location (P=0.801) or the tumor type (P=0.414). The LC rate was significantly higher for tumors ≤35 mm than for tumors >35 mm (92% vs. 54%, P=0.013). The LC rate at 24 months for the 65 peripheral lesions that received 60 Gy as three fractions was 93%, while the 28 central lesions that received 50 Gy as 4 or 5 fractions was 77% (P=0.135).
For the Synchrony® technique, a distance of the target from the fiducial of less than 50 mm improved the LC rate (95% vs. 69%, P=0.011) in bivariate analysis. Of note, only two tumors were >35 mm, so no adjustment for the dimension of the target was performed.
For the Xsight® Spine technique, the LC rate did not differ according to the distance of the target relative to the posterior vertebral wall (P=0.851).
Discussion
This non-randomized cohort study reflects the practice at our institution. It is based on internal guidelines in accordance with the literature, and it is revised at least every three years. Although this study does not have the rigor of a randomized trial, it nonetheless provides useful information.
The objective was to determine the factors influencing local control.
This paper describes the methodology used to evaluate the implementation of the procedure.
In terms of local control, our results are close to what other studies have recently reported in the literature (3,12-14).
Among the analyzed criteria, the tumor type (3,12), primary versus secondary, peripheral versus central tumor, upper vs. lower lobe location, the prescribed dose, and the technique for tracking (15-18) do not influence local control. Differences due to the selected technique would have been found if 239 patients were included (133 more patients with gold seeds and 56 more with coils for a total of 161 patients).
Furthermore, we note that in some cases treatment failures were more common for targets in the lower lobe (P=0.026) and with small tumors localized with Xsight® Spine (13).
It is of interest that the dose level and central versus peripheral situation are strongly correlated. Our protocol, which favors high BED between 110 and 180 Gy may explain similar results.
Two factors were of statistically significant importance:
- A tumor size with a cutoff of 35 mm, which is roughly the limit between T1 and T2 in the AJCC classification. This observation is in keeping with what has been reported by other studies (13,14,19).
- The distance from the target to the fiducials, with a cutoff of 50 mm. Of note, this value is close to the cutoff recommended by the CyberKnife® manufacturer (CyberKnife® technical training program).
Comments regarding the techniques
Gold seeds
We encountered 10 cases of alveolar hemorrhaging. None of these had clinical consequences. Nonetheless, the frequency of pneumothorax underscores the need to exclude patients who are excessively frail. The frequency in our study is consistent with what could be expected (20).
For half of the patients, pneumothorax occurred as early as the implantation procedure, and it prevented the desired number of gold seeds from being implanted. Despite these incidents, the recommended delay of at least seven days between implantation and the treatment planning CT-scan was adhered to (CyberKnife® technical training program), “fiducial placement principles” chapter. The use of bonded seeds allows simultaneous implantation of two seeds, and it eliminates the cases where only one gold seed is available. This could increase the probability of having four markers. In some cases involving paracentral tumors, endobronchial implantation would be an alternative (20).
Endovascular coils
We reserved the use of coils for frail patients whose peripheral tumors preclude the use of the Xspine® technique. As expected, we observed no complications (21,22). The number of fiducials implanted did not appear to be of significant relevance to the probability of local control. Compared to tumors identified by gold seeds, the greater distance to the target could warrant widening of the PTV or abandoning some fiducials. Uncoiling and elongation were frequent occurrences, casting doubt over the accuracy of the tracking. Should the nature of the coil and the type of fiducial placement be important considerations when selecting the provider, the material, or the device?
Improvement of the implantation was possible thanks to acquisition of a new interventional tool. This is a system for interventional imaging which provides a continuous 3D roadmap to guide the procedure (Philips Allura D 20-Clarity, Best, The Netherlands).
When several fiducials are implanted, some may fail to be located, others can have distances between them that exceed our rigid body tolerance (<2.5 mm). Fiducial follow-up may vary during treatment. We found that correcting for rotations was nearly impossible. These data are not reported in the literature. In this series, the significant number of unused fiducials indicates that feedback from radiologists is required to improve the implant geometry.
The LC rate does not appear to be related to the number of fiducials implanted. While it is difficult to increase the number of gold seeds, it is possible to place two gold seeds that are bonded, thus favoring the distance to the number of fiducials.
Xsight® spine
For the Xsight® Spine technique, the preferred maximum distance of the target from the posterior wall (<60 mm) was chosen by analogy with the recommendation for fiducial placement (2006 CyberKnife® Technical training program, Accuray Inc., Sunnyvale, CA, USA). This distance was not always adhered to in patients, probably due to their frailty, their age, or if they were refractory to hospitalization or an invasive procedure. Some cases required enlargement of the margins despite the 4D CT procedure. In fact, we believe that there may be a phase reconstruction artifact with the Xsight® Spine technique and 4D scanner that is responsible for a degree of inaccuracy in the treatment process (23). Indeed, James et al. (24) showed that standard margins of 5 mm on the ITV for patients with lung cancer being treated with stereotactic body radiation therapy are insufficient and that they may result in geographic misses of the tumor when spine tracking is used to locate the position of the tumor in the lung. They therefore recommend the addition of 5 mm margins in all directions for a total of 10 mm to take into account the change in position of the tumor relative to the spine from the time of simulation to treatment (24).
The ITV formed may be incorrectly reconstructed due to technical problems (25) and, as a result, variations owing to the intrafractional target motion can be significant (26).
We did not find that the distance of the target relative to the posterior wall with the Xsight® Spine technique affected the probability of local control. This may be due to the number of included patients or because there was a good selection of patients.
Guo et al. have proposed a maximal distance of 50 mm (13).
As this study shows better local control when the distance of the fiducial to the target is less than 50 mm, we believe that this limit should also be used with the Xspine® technique.
Xsight® lung
Regarding the Xsight® Lung technique, one can hope that the accuracy is as good as possible, although few patients were suitable candidates and the median size of the tumors was larger than in other tracking techniques. Our own experience had shown that below 20 mm, failure to identify the target was a common occurrence. Previous reports have shown that tumors >3.5 cm have ≥80% chance of being adequately visualized (27). As the best results are obtained with smaller tumors, only a limited number of patients underwent this procedure.
The new generation of CyberKnife® and new procedures are more efficient in this regard and they will be evaluated in future studies.
Conclusions
Lung stereotactic radiotherapy allows for a satisfactory level of local control and it represents an alternative to surgical treatment.
Based on our knowledge and for the first time, this study shows that with using the Synchrony® procedure, the best results were obtained when the distance of the target relative to the fiducials was no more than 50 mm, and the required number of fiducials appeared to be two instead of the recommended four.
With appropriate equipment, coils could be a good alternative reserved for the frailest patients.
Other studies ought to be conducted to determine the maximum recommended distance for the Xsight® Spine technique depending on the location of the tumor. It would be useful to identify the weak points of this technique, as it is the most comfortable and the least invasive currently available method.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All patients have been managed in the Institut de Cancérologie de Lorraine according to the standards of good clinical practice. This retrospective study was approved by the local institutional review board and has been declared to the ‘Commission National Informatique et Libertés’ (‘CNIL’), i.e., the French Data Protection Authority.
References
- Tong AN, Yan P, Yuan GH, et al. Advantages of CyberKnife for inoperable stage I peripheral non-small-cell lung cancer compared to three-dimensional conformal radiotherapy. Mol Clin Oncol 2015;3:442-8. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Wang Z, Kong QT, Li J, et al. Clinical outcomes of cyberknife stereotactic radiosurgery for lung metastases. J Thorac Dis 2015;7:407-12. [PubMed]
- Rieber J, Streblow J, Uhlmann L, et al. Stereotactic body radiotherapy (SBRT) for medically inoperable lung metastases-A pooled analysis of the German working group "stereotactic radiotherapy". Lung Cancer 2016;97:51-8. [Crossref] [PubMed]
- Brown WT, Wu X, Fayad F, et al. CyberKnife radiosurgery for stage I lung cancer: results at 36 months. Clin Lung Cancer 2007;8:488-92. [Crossref] [PubMed]
- van der Voort van Zyp NC, Prevost JB, Hoogeman MS, et al. Stereotactic radiotherapy with real-time tumor tracking for non-small cell lung cancer: clinical outcome. Radiother Oncol 2009;91:296-300. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Goldsmith C, Gaya A. Stereotactic ablative body radiotherapy (SABR) for primary and secondary lung tumours. Cancer Imaging 2012;12:351-60. [Crossref] [PubMed]
- Oldrini G, Taste-George H, Renard-Oldrini S, et al. Implantation of fiducial markers in the liver for stereotactic body radiation therapy: Feasibility and results. Diagn Interv Imaging 2015;96:589-92. [Crossref] [PubMed]
- Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 2003;124:1946-55. [Crossref] [PubMed]
- Chang JY, Balter PA, Dong L, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;72:967-71. [Crossref] [PubMed]
- Lischalk JW, Malik RM, Collins SP, et al. Stereotactic body radiotherapy (SBRT) for high-risk central pulmonary metastases. Radiat Oncol 2016;11:28. [Crossref] [PubMed]
- Guo Y, Zhuang H, Zhao L, et al. Influence of different image-guided tracking methods upon the local efficacy of CyberKnife treatment in lung tumors. Thorac Cancer 2015;6:255-9. [Crossref] [PubMed]
- Janvary ZL, Jansen N, Baart V, et al. Clinical Outcomes of 130 Patients with Primary and Secondary Lung Tumors treated with Cyberknife Robotic Stereotactic Body Radiotherapy. Radiol Oncol 2017;51:178-86. [Crossref] [PubMed]
- Kelley KD, Benninghoff DL, Stein JS, et al. Medically inoperable peripheral lung cancer treated with stereotactic body radiation therapy. Radiat Oncol 2015;10:120. [Crossref] [PubMed]
- Jung IH, Song SY, Jung J, et al. Clinical outcome of fiducial-less CyberKnife radiosurgery for stage I non-small cell lung cancer. Radiat Oncol J 2015;33:89-97. [Crossref] [PubMed]
- Temming S, Kocher M, Stoelben E, et al. Risk-adapted robotic stereotactic body radiation therapy for inoperable early-stage non-small-cell lung cancer. Strahlenther Onkol 2018;194:91-7. [Crossref] [PubMed]
- Bibault JE, Prevost B, Dansin E, et al. Image-guided robotic stereotactic radiation therapy with fiducial-free tumor tracking for lung cancer. Radiat Oncol 2012;7:102. [Crossref] [PubMed]
- Ricco A, Davis J, Rate W, et al. Lung metastases treated with stereotactic body radiotherapy: the RSSearch(R) patient Registry's experience. Radiat Oncol 2017;12:35. [Crossref] [PubMed]
- Patel A, Khalsa B, Lord B, et al. Planting the seeds of success: CT-guided gold seed fiducial marker placement to guide robotic radiosurgery. J Med Imaging Radiat Oncol 2013;57:207-11. [Crossref] [PubMed]
- Mongeon M, Thibault F, Chartrand-Lefebvre C, et al. Safety and Efficacy of Endovascular Fiducial Marker Insertion for CyberKnife Stereotactic Radiation Therapy Planning in Early-Stage Lung Cancer. J Vasc Interv Radiol 2017;28:1090-7. [Crossref] [PubMed]
- Karaman K, Dokdok AM, Karadeniz O, et al. Intravascular Placement of Metallic Coils as Lung Tumor Markers for CyberKnife Stereotactic Radiation Therapy. Korean J Radiol 2015;16:626-31. [Crossref] [PubMed]
- Descovich M, McGuinness C, Kannarunimit D, et al. Comparison between target margins derived from 4DCT scans and real-time tumor motion tracking: insights from lung tumor patients treated with robotic radiosurgery. Med Phys 2015;42:1280-7. [Crossref] [PubMed]
- James J, Swanson C, Lynch B, et al. Quantification of planning target volume margin when using a robotic radiosurgery system to treat lung tumors with spine tracking. Pract Radiat Oncol 2015;5:e337-43. [Crossref] [PubMed]
- Li H, Noel C, Garcia-Ramirez J, et al. Clinical evaluations of an amplitude-based binning algorithm for 4DCT reconstruction in radiation therapy. Med Phys 2012;39:922-32. [Crossref] [PubMed]
- Chan MK, Kwong DL, Lee VW, et al. Feasibility study of robotic hypofractionated lung radiotherapy by individualized internal target volume and XSight Spine Tracking: a preliminary dosimetric evaluation. J Cancer Res Ther 2015;11:150-7. [Crossref] [PubMed]
- Bahig H, Campeau MP, Vu T, et al. Predictive parameters of CyberKnife fiducial-less (XSight Lung) applicability for treatment of early non-small cell lung cancer: a single-center experience. Int J Radiat Oncol Biol Phys 2013;87:583-9. [Crossref] [PubMed]


