Independent factors related to preoperative acute lung injury in 130 adults undergoing Stanford type-A acute aortic dissection surgery: a single-center cross-sectional clinical study
Introduction
Acute aortic dissection (AAD) is a serious life-threatening cardiovascular disease (1,2) that frequently results in multiple organ failure, including acute lung injury (ALI) (3,4). The increased risk of ALI in patients with AAD is likely due to a high prevalence of postoperative hypoxemia, as multiple studies have shown that a decreased baseline PaO2/FIO2 ratio is an independent risk factor for postoperative hypoxemia (5-7). Preoperative hypoxemia can not only lead to poor clinical outcomes, but also may postpone surgery to prevent the occurrence of postoperative severe lung injury or acute respiratory disease syndrome (ARDS). Thus, a better understanding of the pathophysiology on the preoperative ALI in patients with AAD can improve clinical prophylaxis and/or treatments. To the best of our knowledge, however, there is no prospective study having assessed the incidence of preoperative ALI and its independent determinants before Stanford type-A AAD surgery in adult patients. In this single-center cross-sectional clinical trial, we observed the incidence of preoperative ALI in adult patients undergoing Stanford type-A AAD surgery and hypothesized that preoperative ALI could be related to clinical and experimental variables.
Methods
Trial design
A single-center cross-sectional clinical trial was used to determine the incidence and factors associated with preoperative ALI in adult patients undergoing Stanford type-A AAD surgery. The study was a prospective single-center registration study and conducted in accordance with a previously described protocol (8), and it has been registered at ClinicalTrials.gov (Identifier: NCT01894334). The study was conducted in accordance with the Declaration of Helsinki, and written informed consent was obtained from all patients and/or immediate relatives.
Ethics
Ethical approval for this study (Identifier: 2012013) was provided by Beijing Anzhen Hospital’s Clinical Research Ethics Committee, Beijing, China (Chairperson Prof. Fang Chen) on August, 1st, 2012.
Study population
The patients with Stanford type-A AAD (1,9) were eligible if they were between 18–75 years of age, and were suitable for emergency surgery. In the recruitment period between January 2013 and July 2014, patients undergoing Stanford type-A AAD surgery at Beijing Anzhen Hospital were enrolled in this study. All of the patients had normal hepatic and renal function. Exclusion criteria included patients with coronary heart disease, severe heart failure, severe cardiac tamponade and severe nervous system abnormalities. Patients prescribed non-steroidal anti-inflammatory drugs or corticosteroids before or after admission were also excluded [available in the inclusion and exclusion criterion in a previously described protocol (8)].
Trial procedure (10,11)
As previously described in the protocol (8), after routine anesthesia and intubation, ALI was identified according to oxygenation index (OI) calculated by PaO2/FiO2 ratio (12), and all the patients were divided into two groups: non-ALI (OI >300 mmHg) and ALI (OI <300 mmHg).
After intubation, the patient’ lungs were mechanically ventilated 10 minutes with a FiO2 100% oxygen, a 5-cmH2O positive end-expiratory pressure (PEEP) and the end-tidal carbon dioxide pressure was maintained at between 35 and 45 mmHg by adjusting tidal volume of 6 to 8 mL/kg and respiratory rate of 10 to 15 beats/min. Then, OI was calculated by arterial blood gas analysis.
Data collection
All of the evaluations were performed and interpreted by an independent expert investigator who was blinded to the initial ALI adjudication. This study protocol was also blinded to patients, serum measurement inspectors, and data statisticians. The data were collected by investigators using collection plans noted in a previously described protocol (8).
Demographic, etiology, hemodynamic, and echocardiogram data were recorded upon admission or after induction. Serum samples of patients in two groups were drawn from a central vein catheter after induction. Plasma levels of interleukin-6 (IL-6), interleukin-10 (IL-10), tissue factor (TF), tissue factor pathway inhibitor (TFPI), human leukocyte elastases (HLE), tumor necrosis factor α (TNF-α), vascular endothelial growth factor (VEGF), prostaglandin I2 (PGI2), and thromboxane B2 (TXB2) were assayed by enzyme-linked immune absorbent assay according to the manufacturer’s specification (MULTISKAN MK3 Automatic microplate reader, Thermo Fisher Scientific Inc., Waltham, MA, USA). Methane dicarboxylic aldehyde (MDA), myeloperoxidase (MPO), total antioxidation capacity (TAOC), and total superoxide dismutase (TSOD) were determined by ultraviolet spectrophotometry (UV-VIS spectrophotometer, Shanghai Jinghua Technology Instruments Co., Ltd., Shanghai, China). The platelet count, hemoglobin (Hb), white blood cells (WBC), lactic acid (LAC), troponin, fibrinogen (FIB), and fibrinogen degradation product (FDP) were determined by standard quantitative assay techniques in the Clinical Laboratory Center of Beijing Anzhen hospital.
Endpoints
The primary endpoint was the protocol-defined incidence of preoperative ALI (8) in before Stanford type-A AAD surgery. The secondary endpoints were independent factors relating to the occurrence of preoperative ALI in adult patients with AAD, which included any necessary factors, such as demographics, etiology, and preoperative assessment of patients.
Statistical analysis
All the data analysis was performed with Empower Stats software (www.empowerstats.com, X&Y Solutions, Inc. Boston, MA, USA) and R software (http://www.R-project.org). Quantitative variables are presented as mean ± standard deviation or median (interquartile range, IQR) and categorical variables as frequencies or percentages. The normally distributed continuous variables were compared by the two-tailed Student t test. Wilcoxon rank sum tests were used for intergroup comparison when parametric data were not normally distributed. The categorical data were compared using Pearson Chi-squared test or Fisher’s exact, as appropriate. Correlations were assessed using Pearson correlation or Spearman’s rank correlation. Variables associated with pre-ALI, which had a P value <0.10, were selected for univariate analysis. Univariate and multiple logistic regression analyses were used to determine the independent factors of preoperative ALI, and estimate the odds ratios and their corresponding 95% CI. Smoothing spline plots of independent factors related to preoperative ALI were created and the threshold effect analysis of independent factors on ALI was performed. P<0.05 was considered to be statistically significant. Receiver operating characteristic (ROC) curves were used to determine the power of multivariable logistic regression model for pre-ALI, and the area under the ROC curve (AUROC) was calculated to assess the discriminative ability of the identified factors for pre-ALI.
Results
Basic characteristics
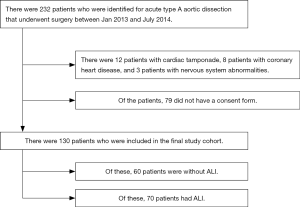
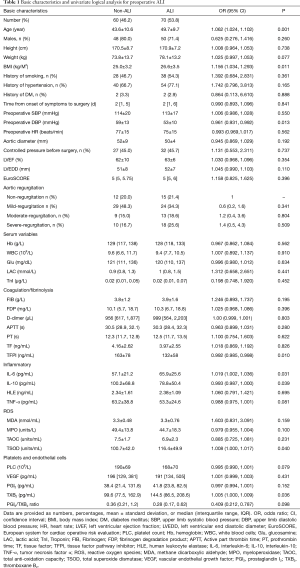
Flow diagram was present in Figure S1. According to inclusion and exclusion criteria, a total of 130 patients undergoing Stanford type-A AAD surgery were ultimately included in this study. The mean age of patients was 46.8 years (ranging from 21 to 72 years), and the majority of patients were male (75.4%). According to the preoperative OI, ALI was identified in 70 patients and the incidence of preoperative ALI was 53.8%. Basic characteristics of patients with and without preoperative ALI are presented in Table 1. The ALI patients were older (OR 1.062, 95% CI, 1.024–1.102; P=0.001), had a higher BMI (OR 1.156, 95% CI, 1.034–1.293; P=0.011), and a lower preoperative DBP (OR 0.961, 95% CI, 0.931–0.992; P=0.013).
Full table
Serum measurements
The plasma TFPI and IL-10 levels were significantly lower in the ALI patients than in the non-ALI patients. Plasma IL-6 and TXB2 levels were significantly increased in the ALI patients compared with the non-ALI patients (Table 1). There were not significant differences in reactive oxygen species (ROS) between the groups.
Univariate analysis and multiple logistic regression analysis for preoperative ALI
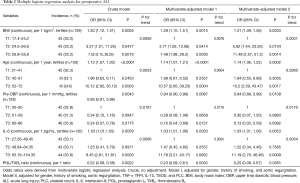
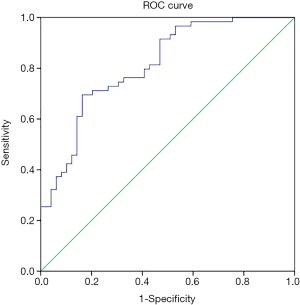
In univariate analysis, 10 preoperative variables, including age, BMI, DBP, platelet count (PLC), TFPI, IL-6, IL-10, TNF-α, TSOD, and PGI2/TXB2 ratio, were associated with preoperative ALI (Table 1). Then, these variables (plus smoking history and aortic regurgitation) were put into the multiple logistic regression analysis, and age, BMI, DBP, IL-6, and PGI2/TXB2 ratio were again shown to be significantly associated with preoperative ALI with or without adjusting for factors (Table 2). Pre-ALI was also consistently related to the tertiles of BMI, age, IL-6, and pre-DBP dose-dependently in the multivariate regression model (Table 2). Receiver-operator characteristic (ROC) curve for the multivariable predictive model of preoperative ALI in patients with AAD was present in Figures S2.
Full table
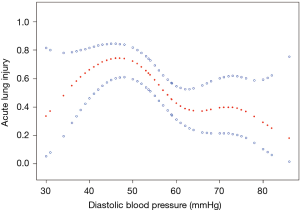
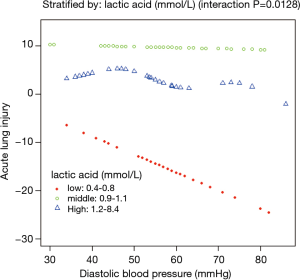
A nonlinear relationship between DBP and ALI was observed (Figure 1). The morbidity of ALI decreased with the preoperative DBP levels up to the turning point (44 mmHg) (OR 0.935, 95% CI, 0.895–0.978; P=0.0033) (Table S1). Interaction analysis revealed that LAC levels completely mediated the relationship between DBP and ALI (P value for interaction, 0.0172). The serum LAC level <0.8 mmol/L had lower ORs between DBP and ALI (OR 0.82; 95% CI, 0.71–0.95; P=0.0065) (Table 3, Figure 2).

Full table

Full table
In sensitivity analysis, main results were not changed using a computer simulation method. Model discrimination was estimated by the ROC curve, and calibration was assessed by bootstrapping with 1,000 replications of individuals sampled with the replacement. Total subjects were divided into two parts, with 75% of cases as modeling data and 25% of cases as validation data, and the AUC (95% CI) for validation data was 0.783 (0.607–0.958, P<0.001) (Table S2). To verify the relationship between blood pressure and ALI, we replaced DBP with lower limb DBP. There was a significant difference in the value of lower limb DBP (58±16 vs. 52±12, P=0.041), but not in the value of lower limb SBP (100±25 vs. 96±25, P=0.395) between the non-ALI and ALI groups. Lower limb DBPs were then put into univariate and multiple logistic regression models (Table S3). Our main results were consistent with the results of DBP.

Full table
Full table
Discussion
In this prospective single-center clinical trial including a sample of 130 adult patients undergoing Stanford type-A AAD surgery, the incidence of preoperative ALI was up to 53.8%, indicating that about half of AAD patients experience preoperative ALI. This finding is consistent with the incidence of preoperative ALI in patients in previous reports (13,14). A retrospective study from Japan showed that oxygenation impairment occurred in 51% of the patients with distal type AAD within 24 h from the onset (13), while a retrospective study from China revealed that 78.49% of the patients undergoing Stanford type-A AAD surgery had the preoperative hypoxemia (14).
The negative influence of preoperative hypoxemia on outcomes after AAD surgery was explored in Wang (14) and colleagues, who reported that preoperative disturbance in oxygenation (PaO2/FiO2 ≤200 mmHg) was associated with a 2.682× increase in the risk of postoperative hypoxemia (PaO2/FiO2 ≤200 mmHg). Postoperative hypoxemia is a dangerous complication and is usually accompanied by prolonged ventilation support and prolonged ICU and hospital stay, which leads to more serious complications with other organs besides the lungs. Moreover, postponed surgery due to severe preoperative hypoxemia increases the risk of aortic rupture, cardiac tamponade, or even death. Therefore, the prophylaxis and/or treatments of preoperative ALI induced by AAD are important for the final outcomes of the patients.
The results of this study confirm our hypothesis that preoperative ALI is related to the clinical and experimental variables. We first observed the independent factors related to preoperative ALI in a series of 130 adult patients, which was helpful in addressing some important underlying mechanisms of preoperative ALI related to AAD. Five variables (age, BMI, preoperative DBP, IL-6, and PGI2/TXB2 ratio) were typically present in Chinese adult patients with preoperative ALI. Our findings were robust even after adjusting the analyses for several important potential confounders.
This study showed that older patients had a higher risk of morbidity due to preoperative ALI after the onset of AAD. Aging increased the risk of thrombotic and inflammatory disorders and intrinsic platelet activation, aggregation, and secretion (15), which were negatively correlated with PLC (r=−0.261, P=0.003). Obese patients may have alterations in pulmonary mechanics to develop ARDS (16,17), and an overproduction of proinflammatory adipokines (18), which was independently associated with morbidity of preoperative ALI. Our data add to the previous publications by showing that age and BMI were related to preoperative ALI in adult patients with AAD.
The AAD mechanism comes from many sources (1,19,20), and recent research has shown that inflammation plays an important role in aortic dissection (21-23). Thus, pulmonary microcirculation is a natural reservoir of leukocytes (24), and is prone to be injury (25). In our study, as we mentioned previously, serum IL-6 was significantly higher in the ALI group, which was an independent factor associated with preoperative ALI in patients with AAD. These data clinically demonstrated that systemic activation of the inflammatory system after AAD plays a key role in the development of ALI.
Platelets also play a crucial role in the pathogenesis of ALI (26), which is activated by AAD to release pro-inflammatory mediators, such as TXA2 (27). As previously reported (3,28,29), Platelets count is reduced in unruptured abdominal aortic aneurysms, and low platelet counts on admission can also predict the increased risk of in-hospital mortality in patients with type A AAD. TXB2 is a stable metabolite of TXA2 (30). In this study, PLC was negatively correlated with TXB2 (r=−0.218, P=0.018). Our results indicated that after the onset of AAD, the production of TXB2 by aggregated platelets was increased in the ALI group, while the balance of the PGI2/TXB2 ratio was interrupted (31). Therefore, the imbalance of the PGI2/TXB2 ratio was an independent factor associated with ALI.
In this study, preoperative DBP was also shown as an independent factor associated with preoperative ALI in the patients with AAD, which was significantly lower in the ALI group. After the onset of AAD, as a rule, the strict control of blood pressure is set by the clinicians to obviously reduce aortic rupture, because the lower the systolic pressure is, the smaller the size of the tear in the false lumen of computational models (1). However, extremely controlled blood pressure, especially controlled DBP, can result in the two serious consequences. One is an increased risk of thrombosis because of lower blood flow within a false lumen, while the other is increased tissue and/or organ malperfusion to prompt release of inflammatory cytokines, which are major factors that cause ALI. In this study, moreover, 77.1% of the patients in the ALI group had a history of hypertension, and tissue and/or organ perfusion would further deteriorate due to induced controlled blood pressure. Therefore, a lower preoperative DBP indicated a higher morbidity of preoperative ALI in patients with AAD. Furthermore, our study also demonstrated that an elevated preoperative DBP was significantly associated with the decreased odds of ALI in patients with AAD. Based on spline smoothing plots and threshold effect analysis of the independent factors on ALI, however, preoperative DBP was non-linearly correlated to ALI. When preoperative DBP was above 44 mmHg, the incidence of preoperative ALI decreased by 6% (according to Model II) by preoperative DBP increasing 1 mmHg. According to stratified analysis by tertiles of preoperative DBP, the incidence of preoperative ALI would decrease to 87% in the patients with preoperative DBP ranging from 60 to 86 mmHg compared to those with preoperative DBP of less than 50 mmHg (according to Model II). In addition, unlike other independent factors, such as age, BMI, IL-6, and PGI2/TXB2 ratio, DBP is a unique modifiable factor that can be modulated by the clinician. It is possible to believe that the value of DBP moving in rational response to fundamentals would be independently associated with the incidence of pre-operative ALI. Our results also raise the question of whether the clinical extent of controlled blood pressure in patients with AAD should be adjusted for the trade-off between the risks of aortic rupture and pre-operative ALI.
Interaction analysis revealed that LAC level played an interactive role in the association between DBP and ALI. When serum LAC level was less than 0.8 mmol/L, a higher DBP would occur and a lower incidence of ALI was noted. Hyperlactatemia is probably a result of both the higher production and lower clearance of LAC due to the pathologic process (32,33). The origin of hyperlactatemia before AAD surgery is multifactorial, and ischemia and reperfusion are important contributors. A lower LAC level indicates a balance of tissue oxygen supply and consumption, in which elevated DBP can improve pulmonary oxygenation by enhancing tissue perfusion and decreasing ischemia and systemic inflammatory response. However, hyperlactatemia indicates a severe unbalance of tissue oxygen supply and consumption, in which elevated amount of DBP cannot compensate such severe unbalance. Our findings suggested that aggressive management to increase DBP and to maintain nearly normal plasma LAC level would be related to decreased risk of ALI before AAD surgery.
In patients with AAD, non-pulmonary factors, including congestive heart failure, can contribute to poor oxygenation. In our study, we excluded patients with left ventricular systolic dysfunction and we also controlled for aortic regurgitation to rule out the contribution of non-pulmonary factors in our results, but our findings persisted. Vasodilators, such as sodium nitroprusside, also reduced PaO2 by increasing ventilation-perfusion mismatching and shunt fraction. However, sodium nitroprusside was used to control systolic blood pressure in 45% of the patients in this study, but both groups were similar in terms of the use of sodium nitroprusside (45% vs. 45.7%). Thus, the use of vasodilators was not an independent factor of oxygenation impairment in this study.
Study limitations
First, this study was only conducted at one medical center, and the number of cases was comparatively small for a relatively short period of patient recruitment. Therefore, a large cohort study is still needed to determine the risk factors of preoperative ALI in the adult patients undergoing Stanford type-A AAD surgery. Second, there was the lack of specificity marker response to lung function, such as the analysis of exhaled breath condensate or specimen of the lung. Third, we were not able to obtain highly detailed information to evaluate the presence of systemic dysfunction in individual cases that required emergency surgery. Forth, while lung function may be deteriorated or improved after mechanical ventilation, OI was calculated based on the respiratory parameters of intubated patients and the score might not be in agreement with the real process. Lastly, OI was used to be equating to a positive outcome, whilst this undoubtedly accounts for the high incidence of ‘ALI’ in the study cohort. However, in ALI group, the OI value of all patients was less than 300 mmHg by having a PEEP ≥5 cmH2O, which defined as acute lung injury according to “The Berlin Definition” (12).
Conclusions
In a sample of 130 adults undergoing Stanford type-A AAD surgery, the incidence of preoperative ALI is 53.8%, and age, BMI, preoperative DBP, IL-6, and PGI2/TXB2 ratio are independent factors associated with pre-operative ALI. Preoperative DBP at more than 44 mmHg is associated with a decreased risk of pre-operative ALI, and LAC mediates the relationship between DBP and ALI before Stanford type-A AAD surgery.
Acknowledgements
All of the authors would like to thank Dr. Fu-Shan Xue, Professor in Department of Anesthesiology, Beijing Friendship Hospital, Capital Medical University, Beijing, China, for his assistance during the investigation and his editorial contributions. All authors thank Prof. Jing Liu, Department of Epidemiology, Beijing Anzhen Hospital, Capital Medical University, Beijing Institute of Heart Lung and Blood Vessel Diseases, Beijing, China, for her kind assistance during the conduct of the statistical analysis and editorial contributions. We also thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding: This study was supported with the grants from Beijing Municipal Science & Technology Commission (No. Z151100004015133 and Z161100000513067), the National Science and Technology Support Program of China (No. 2015BAI12B03), and the Beijing Major Science and Technology Projects from the Beijing Municipal Science and Technology Commission (No. Z171100001017083).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethical approval for this study (Identifier: 2012013) was provided by Beijing Anzhen Hospital’s Clinical Research Ethics Committee, Beijing, China (Chairperson Prof. Fang Chen) on August, 1st, 2012.
References
- Golledge J, Eagle KA. Acute aortic dissection. Lancet 2008;372:55-66. [Crossref] [PubMed]
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. Jama 2000;283:897-903. [Crossref] [PubMed]
- Hasegawa Y, Ishikawa S, Ohtaki A, et al. Impaired lung oxygenation in acute aortic dissection. J Cardiovasc Surg (Torino) 1999;40:191-5. [PubMed]
- Ma WG, Zheng J, Dong SB, et al. Sun's procedure of total arch replacement using a tetrafurcated graft with stented elephant trunk implantation: analysis of early outcome in 398 patients with acute type A aortic dissection. Ann Cardiothorac Surg 2013;2:621-8. [PubMed]
- Weiss YG, Merin G, Koganov E, et al. Postcardiopulmonary bypass hypoxemia: a prospective study on incidence, risk factors, and clinical significance. J Cardiothorac Vasc Anesth 2000;14:506-13. [Crossref] [PubMed]
- Nakajima T, Kawazoe K, Izumoto H, et al. Risk factors for hypoxemia after surgery for acute type A aortic dissection. Surg Today 2006;36:680-5. [Crossref] [PubMed]
- Liu N, Zhang W, Ma W, et al. Risk factors for hypoxemia following surgical repair of acute type A aortic dissection. Interact Cardiovasc Thorac Surg 2017;24:251-6. [PubMed]
- Cheng Y, Jin M, Dong X, et al. Mechanism and early intervention research on ALI during emergence surgery of Stanford type-A AAD: Study protocol for a prospective, double-blind, clinical trial. Medicine (Baltimore) 2016;95. [Crossref] [PubMed]
- Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies. Circulation 2003;108:628-35. [Crossref] [PubMed]
- Sun L, Qi R, Zhu J, et al. Total arch replacement combined with stented elephant trunk implantation: a new "standard" therapy for type a dissection involving repair of the aortic arch? Circulation 2011;123:971-8. [Crossref] [PubMed]
- Sun L, Qi R, Zhu J, et al. Repair of acute type A dissection: our experiences and results. Ann Thorac Surg 2011;91:1147-52. [Crossref] [PubMed]
- Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. Jama 2012;307:2526-33. [PubMed]
- Sugano Y, Anzai T, Yoshikawa T, et al. Serum C-reactive protein elevation predicts poor clinical outcome in patients with distal type acute aortic dissection: association with the occurrence of oxygenation impairment. Int J Cardiol 2005;102:39-45. [Crossref] [PubMed]
- Wang Y, Xue S, Zhu H. Risk factors for postoperative hypoxemia in patients undergoing Stanford A aortic dissection surgery. J Cardiothorac Surg 2013;8:118. [Crossref] [PubMed]
- Mohebali D, Kaplan D, Carlisle M, et al. Alterations in platelet function during aging: clinical correlations with thromboinflammatory disease in older adults. J Am Geriatr Soc 2014;62:529-35. [Crossref] [PubMed]
- Hibbert K, Rice M, Malhotra A. Obesity and ARDS. Chest 2012;142:785-90. [Crossref] [PubMed]
- Gong MN, Bajwa EK, Thompson BT, et al. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax 2010;65:44-50. [Crossref] [PubMed]
- Karnatovskaia LV, Lee AS, Bender SP, et al. Obstructive sleep apnea, obesity, and the development of acute respiratory distress syndrome. J Clin Sleep Med 2014;10:657-62. [PubMed]
- Nienaber CA, Powell JT. Management of acute aortic syndromes. Eur Heart J 2012;33:26-35. [Crossref] [PubMed]
- Wu D, Shen YH, Russell L, et al. Molecular mechanisms of thoracic aortic dissection. J Surg Res 2013;184:907-24. [Crossref] [PubMed]
- Ranasinghe AM, Bonser RS. Biomarkers in acute aortic dissection and other aortic syndromes. J Am Coll Cardiol 2010;56:1535-41. [Crossref] [PubMed]
- Wen D, Du X, Dong JZ, et al. Value of D-dimer and C reactive protein in predicting inhospital death in acute aortic dissection. Heart 2013;99:1192-7. [Crossref] [PubMed]
- Del Porto F, di Gioia C, Tritapepe L, et al. The multitasking role of macrophages in Stanford type A acute aortic dissection. Cardiology 2014;127:123-9. [Crossref] [PubMed]
- Kuebler WM, Goetz AE. The marginated pool. Eur Surg Res 2002;34:92-100. [Crossref] [PubMed]
- Ware LB, Matthay MA. The Acute Respiratory Distress Syndrome. New England Journal of Medicine 2000;342:1334-49. [Crossref] [PubMed]
- Zarbock A, Ley K. The role of platelets in acute lung injury (ALI). Front Biosci (Landmark Ed) 2009;14:150-8. [Crossref] [PubMed]
- Vieira-de-Abreu A, Campbell RA, Weyrich AS, et al. Platelets: versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin Immunopathol 2012;34:5-30. [Crossref] [PubMed]
- Sbarouni E, Georgiadou P, Analitis A, et al. Significant changes in platelet count, volume and size in acute aortic dissection. Int J Cardiol 2013;168:4349-50. [Crossref] [PubMed]
- Huang B, Tian L, Fan X, et al. Low admission platelet counts predicts increased risk of in-hospital mortality in patients with type A acute aortic dissection. Int J Cardiol 2014;172:e484-6. [Crossref] [PubMed]
- Fontana P, Zufferey A, Daali Y, et al. Antiplatelet therapy: targeting the TxA2 pathway. J Cardiovasc Transl Res 2014;7:29-38. [Crossref] [PubMed]
- Shao Y, Cheng Z, Li X, et al. Immunosuppressive/anti-inflammatory cytokines directly and indirectly inhibit endothelial dysfunction- a novel mechanism for maintaining vascular function. Journal of Hematology & Oncology 2014;7:80. [Crossref] [PubMed]
- Garcia-Alvarez M, Marik P, Bellomo R. Stress hyperlactataemia: present understanding and controversy. Lancet Diabetes Endocrinol 2014;2:339-47. [Crossref] [PubMed]
- Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit Care 2014;18:503. [Crossref] [PubMed]