A consensus on the role of osimertinib in non-small cell lung cancer from the AME Lung Cancer Collaborative Group
Introduction
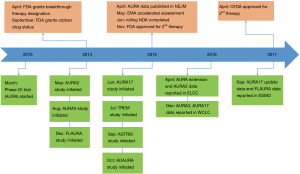
Despite the discovery of driver genes and progress of molecular targeted therapy, non-small cell lung cancer (NSCLC) still remains the leading cause of cancer-related death worldwide (1). Globally, the most common oncogenic driver in NSCLC is the epidermal growth factor receptor (EGFR) in which activating mutations account for approximately 50% of NSCLC in Asian populations and ~15% in Caucasian patients (2-6). Several large-scale phase III clinical trials have consistently demonstrated the superior efficacy of the first or second-generation EGFR tyrosine kinase inhibitors (TKIs) including gefitinib, erlotinib, icotinib and afatinib in patients with EGFR-mutant advanced NSCLC when compared to first-line platinum-based chemotherapy (7-11). In spite of the substantial benefit of 1st and 2nd generation EGFR-TKIs, the vast majority of patients experience disease relapse due to so called acquired resistance within ~1–2 years (3,4,12). The most common mechanism of acquired resistance is the gatekeeper mutation involving the substitution of threonine at position 790 with methionine of EGFR exon 20, known as EGFR T790M mutation (13). It sterically hinders the binding of first-generation EGFR-TKIs to the ATP-binding site of EGFR and is found in more than 50% of acquired resistance cases (4,14,15). This biological insight has facilitated the development of third-generation of EGFR-TKIs. Third-generation EGFR-TKIs are designed to inhibit the function of the EGFR activating and T790M mutations while sparing wild-type EGFR. Hence, they are anticipated to have better efficacy with reduced adverse effects mediated through blockade of the wild type receptor. Osimertinib is a third-generation, central nervous system (CNS)-active EGFR-TKI that potently and selectively inhibits both EGFR sensitizing mutations and EGFR T790M resistance mutations (16,17). It has been approved in a large number of countries (18), including China, the USA, Europe and elsewhere for the treatment of NSCLC patients with acquired resistance to 1st or 2nd generation EGFR-TKIs due to the T790M mutation (12,19-21). More recently, the efficacy of osimertinib in the first-line setting was demonstrated to be clearly superior to standard a first-line treatment in patients with EGFR-mutant NSCLC (22). The current review aims to summary the crucial role of osimertinib in the management of advanced NSCLC (Figure 1).
Overview of osimertinib
Structure and pharmacological features
Osimertinib (AZD9291 or Tagrisso) is a structurally mono-anilino-pyrimidine compound, orally available, third-generation EGFR-TKI that irreversibly binds to the EGFR kinase by targeting the cysteine residue at codon 797 (C797) via covalent bond formation, resulting in a potent, highly selective inhibition (17). In EGFR-recombinant enzyme assays, osimertinib showed potent activity against sensitizing mutations (exon 19 deletion, L858R) and double mutants harboring T790M at a nine-fold lower concentration compared with wild-type EGFR (17,23). Osimertinib is metabolized to produce at least two pharmacologically circulating metabolites, AZ5104 and AZ7550 (17,19,24). AZ7550 showed a similar potency and selectivity to osimertinib and AZ5104 is a more potent inhibitor of exon 19 deletion and T790M mutation and wild-type EGFR than the parent drug. Osimertinib has a broad distribution in tissues, slow absorption and moderate clearance (25). The median time to maximal plasma concentration (Cmax) occurs after 6 h (range, 3–24 h) and steady state is achieved after 15 days of once daily dosing within a 1.6-fold range over the dosing interval (25,26). Plasma concentrations decrease with time and the average half-life is 48 h, with clearance of 14.2 (liter/h). The main metabolic pathways are oxidation and dealkylation and it is eliminated primarily in the feces and urine. Osimertinib is a competitive inhibitor of CYP3A but does not inhibit CYP2C8, 1A2, 2A6, 2B6, 2C9, 2C19, 2D6, and 2E1. Herein strong CYP3A inducers should be avoided during treatment with osimertinib, as concomitant administration may decrease osimertinib plasma concentrations. Pharmacokinetic exposure is not significantly different among distinct ethnicities and food intake does not impact on osimertinib kinetics (24,25).
Preclinical activity
In vitro, osimertinib showed a highly potent inhibitory activity against both sensitizing EGFR mutations (IC50 of 8–17 nmol/L in PC9 harboring exon 19 deletion) and T790M mutation (IC50 of 5–11 nmol/L in H1975 harboring L858R/T790M), with much less activity on wild-type EGFR (IC50 of 650 nmol/L in Calu3 and 461 nmol/L in H2073) (12,17,27,28). In a mouse xenograft model with PC9 or H1975, once-daily dosing of osimertinib induced profound and sustained regression in a dose-dependent manner. Within 5 days of treatment, osimertinib induced significant tumor shrinkage of both EGFR L858R and L858R/T790M tumors. Strikingly, in a comparison between 5 mg/kg/day of osimertinib and 6.25 mg/kg/day of gefitinib, osimertinib induced total regression in all mice and it was sustained during the observation period, while gefitinib induced less tumor regression, and the tumors began to regrow after 90 days after discontinuation (17). Additionally, 25 mg/kg/day of osimertinib daily dosing was well tolerated in the mice even after dosing for 200 days, consistent with osimertinib having a significant selectivity margin over wild-type EGFR in vivo.
With regard to off-target activity, in vitro assays also showed that osimertinib possesses very low activity with a limited number of additional kinases showing greater than 60% inhibition at 1 µmol/L and moderate IC50 potencies for ERBB2, ERBB4, ACK1, ALK, BLK, BBK, MLK1, and MNK2 (17). Although insulin-like growth factor 1 receptor and insulin receptor also have a methionine gatekeeper in their kinase domains, osimertinib and its metabolites (AZ5104 and AZ7550) did not show activity toward this receptor family. This explains why osimertinib resulted in low incidences of hyperglycemia (less than 1%) in clinical trial (29-32).
Clinical efficacy and safety
Second or subsequent line setting
- Osimertinib is the standard therapy for treatment of patients with acquired EGFR T790M mutation after progression on previous EGFR-TKIs (Grade A recommendation, Table 1);
- The detection of EGFR T790M mutation from plasma circulating tumor DNA (ctDNA) samples may guide osimertinib therapy (Grade B recommendation, Table 1).

Full table
On the basis of the preclinical data, several clinical trials were designed to evaluate the clinical efficacy and safety of osimertinib in patients with first-generation EGFR-TKI treated advanced NSCLC and acquired EGFR T790M mutation. The first trial was the phase I dose-escalation and expansion parts of Osimertinib First Time in Patients Ascending Dose (AURA), an open-label, multicenter study. The AURA study enrolled 253 patients with advanced NSCLC and EGFR sensitizing mutations, who had disease progression on prior EGFR-TKIs (31). For 127 patients with center-confirmed T790M mutation, the objective response rate (ORR) was 61%, and progression-free survival (PFS) was 9.6 months. The most common adverse events (AEs) were rash, diarrhea, nausea, and decreased appetite with only a few serious AEs. In the subsequent phase II trial (AURA2), 210 NSCLC patients with activating EGFR mutations and confirmed T790M after progression on prior EGFR-TKIs were included (osimertinib: 80 mg, once daily) and the ORR was 71% (30). In a recent pooled analysis (n=411) that included AURA extension and AURA2 NSCLC patients with centrally confirmed T790M mutation after progression on previous EGFR-TKIs, the ORR was 66% and PFS was 11.0 months.
The phase III clinical trial, AURA3, further demonstrated the superior efficacy of osimertinib in patients with EGFR T790M-mutant advanced NSCLC (32). In this open-label, randomized phase III trial, 419 patients with EGFR T790M-mutant advanced NSCLC and disease progression after first-line EGFR-TKIs were randomly assigned to receive oral osimertinib (at a dose of 80 mg once daily), or intravenous pemetrexed plus carboplatin or cisplatin for up to six cycles in a 2:1 ratio (32). Maintenance pemetrexed and switch to osimertinib following disease progression on chemotherapy were permitted. The primary end point was PFS. As the results show, the median PFS was significantly longer for patients treated with osimertinib compared with chemotherapy [10.1 vs. 4.4 months, hazard ratio (HR) 0.30, P<0.001]. The ORR was also significantly better with osimertinib (71%) than with platinum-based chemotherapy (31%). Furthermore, among 144 patients with CNS metastases, the median PFS was also markedly longer among patients receiving osimertinib than among those receiving chemotherapy (8.5 vs. 4.2 months; HR 0.32) (32). These results prompted the FDA to approve osimertinib for the treatment of patients with acquired EGFR T790M mutation after progression on previous EGFR-TKIs (33).
First-line setting
- Osimertinib is the appropriate strategy for the first-line treatment of patients with EGFR activating mutation (Grade A recommendation, Table 1).
The AURA study included two cohorts of patients, those who had received osimertinib as first-line treatment of EGFR-mutant advanced NSCLC (treatment-naïve) and those who had experienced disease progression on prior EGFR-TKIs. In the treatment-naïve group, sixty patients with locally advanced or metastatic EGFR-mutant NSCLC received osimertinib once daily (n=30, 80 mg; n=30, 160 mg). The primary endpoint was ORR, PFS and safety assessment (22). With a median follow-up of 19.1 months, the ORR was 77% (67% in the 80-mg group; 87% in the 160-mg group). The median PFS was 20.5 months (22.1 months in 80-mg group; 19.3 months in 160-mg group). The median PFS was longer in patients with exon 19 deletion than those with L858R mutation or other mutations (23.4 vs. 22.1 vs. 8.3 months). All the included patients experienced AEs and 62% of them experienced grade ≥3 AEs. Notably, one patient experienced an AE leading to death, but was not causally related to osimertinib (22).
At the 2017 European Society for Medical Oncology (ESMO) Annual Meeting, Ramalingam et al. reported the phase III randomized study that assessed the efficacy and safety of osimertinib versus first-generation EGFR-TKIs in first-line patients with advanced NSCLC and sensitizing EGFR mutations (FLAURA). Globally, 556 patients from Asia, Europe, and North America were randomized 1:1 to osimertinib 80 mg once daily, orally or standard of care EGFR-TKI (gefitinib 250 mg or erlotinib 150 mg once daily, orally). The median PFS was 18.9 months with osimertinib compared to 10.2 months for the standard therapy, with an HR of 0.46 (95% confidence interval, 0.37–0.57; P<0.0001). PFS benefit was consistent across all subgroups, including patients with and without brain metastases (BMs) at initial therapy. The median duration of response was 17.2 months in patients treated with osimertinib compared to 8.5 months in the standard of care group. The ORR was 80% with osimertinib compared to 76% with standard therapy. Strikingly, overall survival (OS) appeared to favor osimertinib with a HR of 0.63 although this was not statistically significant at the interim OS analysis (25% maturity). With regard to toxicities, the incidence of grade 3 or higher AEs was lower for osimertinib (34%) than the standard therapy (45%). The most common AEs were diarrhea (58%) and dry skin (32%) in the osimertinib group and diarrhea (57%) and dermatitis acneiform (48%) in the standard treatment group.
Efficacy on CNS metastases
- Osimertinib is the optional strategy for the treatment of NSCLC patients with EGFR mutation and CNS metastases (Grade B recommendation, Table 1).
Lung cancer is characterized by a high incidence of CNS metastases, with BMs developing in approximately 40% of patients at some time during the course of the disease (34,35). Patients with NSCLC and CNS metastasis often have a dismal prognosis (36,37). The first-generation EGFR-TKIs have poor penetration of the blood-brain barrier (BBB). Although erlotinib had the better penetration rate than gefitinib, both barely reached a satisfactory effect for NSCLC patients with CNS metastasis (38-41). In the mouse model, osimertinib had a 10-fold greater distribution rate into the brain than gefitinib (42). Osimertinib may also significantly reduce brain lesions. These data have been further validated by Ballard et al. who confirmed a greater penetration of osimertinib across the BBB than gefitinib, afatinib and rociletinib in a mouse model (43). Consistent with these findings, the clinical activity of osimertinib has been characterized in patients with CNS metastasis. A pooled analysis of patients from AURA and AURA2 found that the systemic ORR was 61%, while the ORR of patients with and without CNS metastases was 56% and 64%, respectively (44). At the 2017 American Society of Clinical Oncology (ASCO) meeting, subgroup analysis from AURA3 showed that the confirmed CNS ORRs by neuroradiologist blinded independent central review assessed (BICR) in the osimertinib and platinum-based groups were 70% and 31%, respectively. The disease control rate (DCR) was also markedly higher in the osimertinib group than in the platinum-based group (87% vs. 68%). In patients with measurable or non-measurable baseline CNS lesions, CNS PFS by neuroradiologist BICRs were significantly prolonged with osimertinib therapy versus platinum-based therapy (11.7 vs. 5.6 months; P=0.004). The intracranial response rate in the AURA extension and AURA2 studies was 68% (49% to 83%) with 24% complete intracranial responses (45). In the FLAURA study, the intracranial response rate was 66% and in patients without prior radiation was 91% with an intracranial complete response (CR) rate of 23–25%. The intracranial DCR was 90% (80–96%) (Vansteenkiste et al., 2017 ESMO Asia).
Additionally, osimertinib was demonstrated as an effective treatment for 1st or 2nd generation EGFR-TKI-resistant leptomeningeal carcinomatosis caused by EGFR-mutant lung cancer in vivo (46). Osimertinib is currently being evaluated for the treatment of NSCLC patients with leptomeningeal and brain metastasis in a phase I study (BLOOM; NCT02228369). The preliminary results with 20 patients showed that 7 had radiological improvement, 2 had a stable disease and 3 were not evaluable in 12 patients reaching the 12-week neurological assessment. Among these patients, 6 were symptomatic, of which 3 had improvement in neurological symptoms, while 1 had no change and 2 were not evaluable (47). Collectively, these data favor the hypothesis that osimertinib does penetrate the BBB and is effective against CNS metastases from EGFR-mutant NSCLC. However, these findings are still immature and should be interpreted with caution.
Safety and tolerability
- The safety and tolerability of osimertinib is superior to first and second-generation EGFR-TKIs (Grade A- recommendation, Table 1).
The major AEs of the currently available 1st and 2nd generation EGFR-TKIs (gefitinib, erlotinib, icotinib and afatinib) are due to the inhibition of wild-type EGFR in the skin and gastrointestinal tract. However, osimertinib is a highly selective inhibitor of EGFR sensitizing or T790M mutations, while sparing wild-type EGFR, showing around 200-fold greater potency against L858R/T790M than wild-type EGFR (17). Based on this mechanism of action, osimertinib was well tolerated across phase I to III trials in comparison with previous EGFR-TKIs. In the phase I dose-escalation AURA study, osimertinib showed good tolerability and no dose-limiting toxicities (DLT) were observed even at the largest dose level (240 mg, once daily) (31). Grade of AEs were observed in 96% of all cases, with 32% of patients experiencing grade 3–5 AEs. The most common AEs were diarrhea (47%), rash (40%), nausea (22%), and decreased appetite (21%). AEs leading to dose reduction or drug discontinuation were observed in 7% and 6%, respectively. Treatment-related serious AEs, as assessed by the site investigator, occurred in 6% of patients. There were no significant differences in the severity or frequency of AEs between different ethnicities. Notably, 2.4% of cases suffered from pneumonitis-like AEs, resulting in osimertinib discontinuation. In addition, 11 patients (4.3%) developed prolongation of the QTc interval while six patients (2.4%) experienced hyperglycemia during osimertinib treatment. There were 7 fatal AEs, one of which (pneumonia) was reported as being possibly drug-related. Similar results were found in the AURA extension cohorts and AURA2 study. In the pooled analysis of these trials, the most common AEs were diarrhea (42%), rash (41%), dry skin (31%) and paronychia (25%) (48). AE-related drug dose reduction or discontinuation was observed in 4.4% and 5.6% of all cases. Remarkably, four patients suffered from fatal interstitial lung disease confirmed by the investigator (29,30). In the phase III AURA3 study, 273 of 279 (98%) patients in the osimertinib group experienced one of the grades of AE. There were fewer reported cases of AEs grade ≥3 in the osimertinib group than in the chemotherapy group (23% vs. 47%). The most common AEs in the osimertinib group were similar to those reported in the pooled analysis of AURA extension and AURA2 study including diarrhea (41%), rash (34%), dry skin (23%) and paronychia (22%). Interstitial lung disease-like AEs were observed in 4% of cases, with 9 events of grade 1 or 2 in severity and 1 death. A prolongation in the QT interval was reported in 10 cases. Osimertinib was associated with a lower rate of AEs leading to permanent discontinuation in comparison with chemotherapy (7% vs. 10%) (32). Taken together, although long-term follow-up is required for accurate evaluation on safety and tolerability, it seems that osimertinib has a more acceptable toxicity profile and tolerability than first- and second-generation EGFR-TKIs. The AEs in first-line treatment were similar to those in the previous trials. In FLAURA, 91% of patients experienced treatment-related AEs, with 49 cases (18%) of grade ≥3 AEs. The most common AEs were diarrhea (58%) and dry skin (32%) in the osimertinib group. However, osimertinib was associated with a lower rate of AEs leading to permanent discontinuation compared to first-generation EGFR-TKIs (13.3% vs. 18.1%).
Future directions
Acquired resistance and overcoming strategies
With the success of AURA3 and FLAURA, there is no doubt that osimertinib may become the standard of care for patients with EGFR activating mutations and no prior treatment, or EGFR T790M mutation-positive NSCLC who have progressed on previous EGFR-TKI treatment. Nevertheless, several future directions of osimertinib should be addressed. Firstly, despite the superior efficacy of osimertinib in both sensitizing EGFR mutation and EGFR T790M mutation, drug resistance is still inevitable. To date, a number of studies have reported on resistance mechanisms (49,50). Analogous to the catalog of resistance mechanisms to first-generation EGFR-TKIs, we can also put the reported resistance mechanism into several groups: (I) secondary mutations or amplification of the EGFR gene (51-57); (II) alternative pathway activation (58-62); (III) histological transformation (62). A retrospective analysis on serial ctDNA from patients treated with osimertinib revealed that a tertiary acquired EGFR C797S mutation may be one of the main mechanisms (50,52). Our group also reported a novel mutation on EGFR Leu792 correlating with acquired resistance to osimertinib (56). At the 2017 ASCO, Zofia et al. reported a cohort of 23 osimertinib-acquired resistance patients with extensive pre/post-osimertinib tissue and plasma. Their findings suggested that MET amplification (7/23; 30%) and EGFR C797S (5/23; 22%) were the most common mechanisms. Another mechanism included small-cell lung cancer transformation, PIK3CA E545K/PIK3CA amplification and fibroblast growth factor receptor 1 (FGFR1) D60N/FGFR1 amplification. Interestingly, two recent studies explored new approaches in overcoming the C797S mutation and found a promising antitumor effect in the mouse model (63,64). However, whether these strategies could benefit patients with resistance to osimertinib is not yet known.
Liquid biopsy for EGFR T790M detection and dynamic monitoring
Post-hoc analysis of the AURA trial showed that ORR and median PFS were similar in patients with T790M-positive plasma or T790M-positive tumor (ORR: 63% vs. 62%; PFS: 9.7 vs. 9.7 months), supporting the clinical utility of detecting EGFR T790M mutation from plasma ctDNA samples (65). These results provided evidence for the application of ctDNA as a reliable alternative to tumor re-biopsy for testing T790M status. On the basis of this, the FDA recently approved blood-based EGFR mutation testing for osimertinib therapy. However, patients with T790M-negative plasma had a 46% of ORR and 8.2 months of PFS, suggesting that patients with T790M negative plasma could benefit from osimertinib therapy and still need a tumor re-biopsy to test for the presence of T790M mutation (65). Analogously, previous studies reported that the sensitivity was approximately 60% for detecting the EGFR T790M mutation in a plasma-based test, which was lower than for the EGFR sensitizing mutation (70–80%) (66-70). This result suggests that more than half of all patients with T790M-mutant NSCLC could avoid an invasive biopsy with the application of a plasma-based test, while patients with T790M-negative plasma results should still be advised to receive tissue-based EGFR testing due to the high false-negative rate of the plasma-based detection. In addition, recent studies also suggested that plasma ctDNA can be used to dynamically monitor the therapeutic effect and explore the acquired resistance mechanisms to osimertinib (22,52,56). In treatment-naïve patients with EGFR-mutant NSCLC in the AURA study, patients with undetectable ctDNA had a significantly longer mean time to RECIST-defined progression than those with detectable ctDNA at presentation (19.6 vs. 13.1 months). The mean time to osimertinib discontinuation was also longer in patients with undetectable ctDNA than in patients with detectable ctDNA (25.2 vs. 18.2 months), suggesting that detectable ctDNA may be useful in the future management of osimertinib treatment. This study also suggested that ctDNA is available for the exploration of acquired resistance mechanisms to osimertinib. In 19 patients with detectable ctDNA, 9 of them had putative genomic resistance mutations including EGFR C797S mutations (n=2), MET amplification (n=1), EGFR and KRAS amplification (n=1), MEK1, KRAS, PIK3CA, JAK2 mutation (n=1 each), and HER2 exon 20 insertion (n=1) (22). Collectively, the application of liquid biopsy will play a significant role in the future long-term management of patients with NSCLC and EGFR mutations.
Efficacy on EGFR exon 20 insertion mutations
EGFR exon 20 insertion mutations, which are typically located after the C-helix of the tyrosine kinase domain of EGFR, may account for ~4% of all EGFR mutations (71). Preclinical studies have shown that most EGFR exon 20 insertion mutations, except for ex20insFQEA are resistant to 1st (gefitinib, erlotinib) and 2nd (neratinib and afatinib) generation EGFR-TKIs (72). To date, there is no effective inhibitor on EGFR exon 20 insertion mutations, resulting in a dismal survival in patients with an EGFR exon 20 insertion mutation (73). In recent preclinical studies, osimertinib showed potent efficacy on some forms of exon 20 insertion mutations including Y764_V765insHH, A767_V769dupASV, and D770_N771insNPG (28). Furthermore, osimertinib showed 3–20-fold lower IC50 values in exon 20 insertion mutations in comparison with wild type, while afatinib showed 1.5–5-fold higher values in exon 20 insertion mutations to wild type. These results suggest that osimertinib had a wider selectivity margin for several exon 20 insertion mutations over wild type EGFR than afatinib. However, compared to sensitizing EGFR mutations with or without T790M mutations, exon 20 insertion mutations had 10–100-fold IC50 values of osimertinib. Therefore, a higher dose of osimertinib (>80 mg; once daily) will be necessary to effectively treat patients with EGFR exon 20 insertion mutations. Notably, a recent study showed that poziotinib, a potent, clinically active inhibitor of EGFR and HER2 exon 20 mutations, demonstrated greater activity than approved EGFR TKIs in vitro and in patient-derived xenograft models of EGFR or HER2 exon 20 mutant NSCLC and in genetically engineered mouse models of NSCLC (74). In a phase II trial, the first 11 patients with NSCLC with EGFR exon 20 mutations receiving poziotinib had a confirmed ORR of 64%, suggesting its potential application in these patients (74).
Activity for CNS metastases
As previously mentioned, lung cancer has a high incidence (~40%) of CNS metastasis with a dismal prognosis and limited therapeutic strategies (34). Osimertinib has shown clinical activity in patients with CNS metastasis. In the AURA3 study, Mok et al. highlighted that “a key finding is that even patients with CNS metastasis can benefit from osimertinib” (33). Similarly, in FLAURA, the results indicated that osimertinib could bring PFS benefit for patients with and without BMs, suggesting that osimertinib is active in the brain as well as in systemic sites. If this encouraging result is demonstrated in future appropriately designed, prospective study, it will shift the management of patients with EGFR mutant-NSCLC and CNS metastasis. An open label, phase III study aimed to investigate the efficacy and safety of osimertinib in BMs from patients with EGFR T790M positive NSCLC who have received prior therapy with an EGFR-TKI is ongoing (NCT02972333). Previous studies have attempted to delay the use of radiation treatment in the management of patients with EGFR-mutant lung cancer. Importantly, Magnuson et al demonstrated that delay in radiotherapy was associated with inferior survival, while giving stereotactic radiosurgery (SRS) followed by EGFR-TKI was associated with an improved survival (HR 0.39, P<0.001) (75). The excellent intracranial control noted in the AURA and FLAURA trials will help guide management of patients with BM and EGFR mutation; calling into question the utility once again of upfront use of whole brain radiation therapy (WBRT) or SRS. Additional prospective studies are needed addressing these questions.
Combination therapy
To further improve the efficacy of osimertinib, distinct combinatorial strategies including combination with immune checkpoint inhibitors, bevacizumab, dasatinib and others, are now under investigation. In a phase Ib study, the researchers investigated the combination of osimertinib and the anti-PD-L1 antibody durvalumab in pretreated EGFR-mutant NSCLC patients with or without T790M mutation (NCT02143466). The preliminary results showed that 52% of evaluable patients obtained a partial response. However, the high incidence of interstitial lung disease and diarrhea in the combination group remains a challenging issue. Osimertinib is also being investigated in the combination with bevacizumab for the treatment of EGFR TKI-naïve patients with EGFR mutation (NCT02803203). The primary result is anticipated to be reported in June 2019. Recently, a preclinical study revealed that Cripto-1 overexpression was an intrinsic resistance mechanism to EGFR-TKIs via activation of the Src oncogene (76). Based on this, a study aimed to evaluate the efficacy of osimertinib plus dasatinib, an BAL1/SRC TKI, in patients with EGFR-mutant NSCLC is ongoing (NCT02954523).
Role in adjuvant therapy
The role of EGFR-TKIs as adjuvant treatment for patients with EGFR-mutant stage Ib–IIIa NSCLC after surgery remains controversial (77-82). In a recent meta-analysis, the authors analysed five studies to assess whether EGFR-TKIs could improve the outcomes of patients with NSCLC after complete resection. The pooled results showed that adjuvant EGFR-TKIs could significantly prolong disease-free survival and reduce the risk of distant metastasis in patients with EGFR-mutant NSCLC after complete resection (79). Hence, it is worthwhile to further investigate the clinical value of third-generation EGFR-TKIs in the adjuvant setting. The ADAURA trial is a double-blind, placebo-controlled randomized study evaluating the efficacy and safety of osimertinib versus placebo as adjuvant treatment for patients with EGFR-mutant stage Ib–IIIa NSCLC who underwent complete tumor resection followed or not followed by adjuvant chemotherapy (NCT02511106). This study is currently recruiting participants.
Conclusions
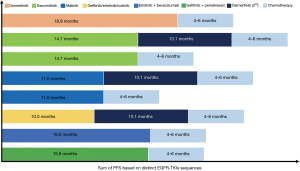
To date, osimertinib is one of the most promising and effective third-generation EGFR-TKIs in patients with EGFR sensitizing mutations with or without EGFR T790M mutation. The success of AURA3 prompts final approval of osimertinib for treatment of NSCLC patients with EGFR T790M mutation who had progressed on previous EGFR-TKI therapy. Recently, the efficacy of osimertinib in the first-line setting was demonstrated in the FLAURA trial with clear superiority over first-generation EGFR-TKIs in treatment-naïve patients with EGFR-mutant NSCLC. However, the optimal sequence of distinct generation EGFR-TKIs in the long-term management of patients with EGFR-mutant NSCLC remains controversial (Figure 2). In addition, there are several unresolved issues on osimertinib including acquired resistance mechanisms, the application of plasma ctDNA for testing EGFR T790M mutation, its efficacy in patients with CNS metastases or exon 20 mutations, its combination with other therapeutic strategies and its role in adjuvant therapy that should be emphasized in future clinical investigation.
Acknowledgements
Funding: This study was sponsored by the National Natural Science Foundation of China (No. 81672286 and 81772467), “Shuguang Program” supported by Shanghai Education Development Foundation and Shanghai Municipal Education Commission (No. 16SG18), and the Outstanding Young Doctor Program of the Shanghai Municipal Commission of Health and Family Planning (No. XYQ2013097).
Footnote
Conflicts of Interest: NA Pennell has done consulting for AstraZeneca which makes osimertinib. The other authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154-62. [Crossref] [PubMed]
- Hirsch FR, Suda K, Wiens J, et al. New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet 2016;388:1012-24. [Crossref] [PubMed]
- Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol 2014;11:473-81. [Crossref] [PubMed]
- Cancer Genome Atlas Research N. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543-50. [Crossref] [PubMed]
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016;387:1415-26. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [Crossref] [PubMed]
- Soejima K, Yasuda H, Hirano T. Osimertinib for EGFR T790M mutation-positive non-small cell lung cancer. Expert Rev Clin Pharmacol 2017;10:31-8. [Crossref] [PubMed]
- Shih JY, Gow CH, Yang PC. EGFR mutation conferring primary resistance to gefitinib in non-small-cell lung cancer. N Engl J Med 2005;353:207-8. [Crossref] [PubMed]
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240-7. [Crossref] [PubMed]
- Tan CS, Gilligan D, Pacey S. Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer. Lancet Oncol 2015;16:e447-59. [Crossref] [PubMed]
- Jiang T, Zhou C. Clinical activity of the mutant-selective EGFR inhibitor AZD9291 in patients with EGFR inhibitor-resistant non-small cell lung cancer. Transl Lung Cancer Res 2014;3:370-2. [PubMed]
- Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046-61. [Crossref] [PubMed]
- Greig SL. Osimertinib: First Global Approval. Drugs 2016;76:263-73. [Crossref] [PubMed]
- Lamb YN, Scott LJ. Osimertinib: A Review in T790M-Positive Advanced Non-Small Cell Lung Cancer. Target Oncol 2017;12:555-62. [Crossref] [PubMed]
- Ricciuti B, Chiari R, Chiarini P, et al. Osimertinib (AZD9291) and CNS Response in Two Radiotherapy-Naive Patients with EGFR-Mutant and T790M-Positive Advanced Non-Small Cell Lung Cancer. Clin Drug Investig 2016;36:683-6. [Crossref] [PubMed]
- Gao X, Le X, Costa DB. The safety and efficacy of osimertinib for the treatment of EGFR T790M mutation positive non-small-cell lung cancer. Expert Rev Anticancer Ther 2016;16:383-90. [Crossref] [PubMed]
- Ramalingam SS, Yang JC, Lee CK, et al. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:841-9. [Crossref] [PubMed]
- Finlay MR, Anderton M, Ashton S, et al. Discovery of a potent and selective EGFR inhibitor (AZD9291) of both sensitizing and T790M resistance mutations that spares the wild type form of the receptor. J Med Chem 2014;57:8249-67. [Crossref] [PubMed]
- Sullivan I, Planchard D. Osimertinib in the treatment of patients with epidermal growth factor receptor T790M mutation-positive metastatic non-small cell lung cancer: clinical trial evidence and experience. Ther Adv Respir Dis 2016;10:549-65. [Crossref] [PubMed]
- Planchard D, Brown KH, Kim DW, et al. Osimertinib Western and Asian clinical pharmacokinetics in patients and healthy volunteers: implications for formulation, dose, and dosing frequency in pivotal clinical studies. Cancer Chemother Pharmacol 2016;77:767-76. [Crossref] [PubMed]
- Ricciuti B, Baglivo S, Paglialunga L, et al. Osimertinib in patients with advanced epidermal growth factor receptor T790M mutation-positive non-small cell lung cancer: rationale, evidence and place in therapy. Ther Adv Med Oncol 2017;9:387-404. [Crossref] [PubMed]
- Walter AO, Sjin RT, Haringsma HJ, et al. Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes T790M-mediated resistance in NSCLC. Cancer Discov 2013;3:1404-15. [Crossref] [PubMed]
- Hirano T, Yasuda H, Tani T, et al. In vitro modeling to determine mutation specificity of EGFR tyrosine kinase inhibitors against clinically relevant EGFR mutants in non-small-cell lung cancer. Oncotarget 2015;6:38789-803. [Crossref] [PubMed]
- Yang JC, Ahn MJ, Kim DW, et al. Osimertinib in Pretreated T790M-Positive Advanced Non-Small-Cell Lung Cancer: AURA Study Phase II Extension Component. J Clin Oncol 2017;35:1288-96. [Crossref] [PubMed]
- Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016;17:1643-52. [Crossref] [PubMed]
- Janne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Hutchinson L. Lung cancer: AURA3 magic reveals new standard. Nat Rev Clin Oncol 2017;14:69. [Crossref] [PubMed]
- Jiang T, Su C, Li X, et al. EGFR TKIs plus WBRT Demonstrated No Survival Benefit Other Than That of TKIs Alone in Patients with NSCLC and EGFR Mutation and Brain Metastases. J Thorac Oncol 2016;11:1718-28. [Crossref] [PubMed]
- Lombardi G, Di Stefano AL, Farina P, et al. Systemic treatments for brain metastases from breast cancer, non-small cell lung cancer, melanoma and renal cell carcinoma: an overview of the literature. Cancer Treat Rev 2014;40:951-9. [Crossref] [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25. [Crossref] [PubMed]
- Eichler AF, Kahle KT, Wang DL, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol 2010;12:1193-9. [Crossref] [PubMed]
- Lee E, Keam B, Kim DW, et al. Erlotinib versus gefitinib for control of leptomeningeal carcinomatosis in non-small-cell lung cancer. J Thorac Oncol 2013;8:1069-74. [Crossref] [PubMed]
- Brower JV, Robins HI. Erlotinib for the treatment of brain metastases in non-small cell lung cancer. Expert Opin Pharmacother 2016;17:1013-21. [Crossref] [PubMed]
- Porta R, Sanchez-Torres JM, Paz-Ares L, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J 2011;37:624-31. [Crossref] [PubMed]
- Park SJ, Kim HT, Lee DH, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer 2012;77:556-60. [Crossref] [PubMed]
- Remon J, Planchard D. AZD9291 in EGFR-mutant advanced non-small-cell lung cancer patients. Future Oncol 2015;11:3069-81. [Crossref] [PubMed]
- Ballard P, Yates JW, Yang Z, et al. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin Cancer Res 2016;22:5130-40. [Crossref] [PubMed]
- Ahn MJ, Tsai CM, Yang JC, et al. AZD9291 activity in patients with EGFR-mutant advanced non-small cell lung cancer (NSCLC) and brain metastases: data from Phase II studies Eur J Cancer 2015;51:S625-6. [Abstract #3083]. [Crossref]
- Goss G, Tsai CM, Shepherd FA, et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: pooled data from two phase II trials. Ann Oncol 2018;29:687-93. [Crossref] [PubMed]
- Nanjo S, Ebi H, Arai S, et al. High efficacy of third generation EGFR inhibitor AZD9291 in a leptomeningeal carcinomatosis model with EGFR-mutant lung cancer cells. Oncotarget 2016;7:3847-56. [Crossref] [PubMed]
- Yang JC, Kim DW, Kim SW, et al. Osimertinib activity in patients (pts) with leptomeningeal (LM) disease from non-small cell lung cancer (NSCLC): updated results from BLOOM, a phase I study. J Clin Oncol 2016.34. abstract 9002.
- Yang J, Ramalingam SS, Jänne PA, et al. Osimertinib (AZD9291) in pre-treated patients with T790M-positive advanced NSCLC: updated phase I and pooled phase II results. J Thorac Oncol 2016;11:S152-3. [Crossref] [PubMed]
- Wang S, Song Y, Yan F, et al. Mechanisms of resistance to third-generation EGFR tyrosine kinase inhibitors. Front Med 2016;10:383-8. [Crossref] [PubMed]
- Wang S, Tsui ST, Liu C, et al. EGFR C797S mutation mediates resistance to third-generation inhibitors in T790M-positive non-small cell lung cancer. J Hematol Oncol 2016;9:59. [Crossref] [PubMed]
- Knebel FH, Bettoni F, Shimada AK, et al. Sequential liquid biopsies reveal dynamic alterations of EGFR driver mutations and indicate EGFR amplification as a new mechanism of resistance to osimertinib in NSCLC. Lung Cancer 2017;108:238-41. [Crossref] [PubMed]
- Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015;21:560-2. [Crossref] [PubMed]
- Zheng D, Hu M, Bai Y, et al. EGFR G796D mutation mediates resistance to osimertinib. Oncotarget 2017;8:49671-9. [PubMed]
- Ou SI, Cui J, Schrock AB, et al. Emergence of novel and dominant acquired EGFR solvent-front mutations at Gly796 (G796S/R) together with C797S/R and L792F/H mutations in one EGFR (L858R/T790M) NSCLC patient who progressed on osimertinib. Lung Cancer 2017;108:228-31. [Crossref] [PubMed]
- Oztan A, Fischer S, Schrock AB, et al. Emergence of EGFR G724S mutation in EGFR-mutant lung adenocarcinoma post progression on osimertinib. Lung Cancer 2017;111:84-7. [Crossref] [PubMed]
- Chen K, Zhou F, Shen W, et al. Novel Mutations on EGFR Leu792 Potentially Correlate to Acquired Resistance to Osimertinib in Advanced NSCLC. J Thorac Oncol 2017;12:e65-8. [Crossref] [PubMed]
- Bersanelli M, Minari R, Bordi P, et al. L718Q Mutation as New Mechanism of Acquired Resistance to AZD9291 in EGFR-Mutated NSCLC. J Thorac Oncol 2016;11:e121-3. [Crossref] [PubMed]
- Ho CC, Liao WY, Lin CA, et al. Acquired BRAF V600E Mutation as Resistant Mechanism after Treatment with Osimertinib. J Thorac Oncol 2017;12:567-72. [Crossref] [PubMed]
- Ou SI, Agarwal N, Ali SM. High MET amplification level as a resistance mechanism to osimertinib (AZD9291) in a patient that symptomatically responded to crizotinib treatment post-osimertinib progression. Lung Cancer 2016;98:59-61. [Crossref] [PubMed]
- Ichihara E, Westover D, Meador CB, et al. SFK/FAK Signaling Attenuates Osimertinib Efficacy in Both Drug-Sensitive and Drug-Resistant Models of EGFR-Mutant Lung Cancer. Cancer Res 2017;77:2990-3000. [Crossref] [PubMed]
- Planchard D, Loriot Y, Andre F, et al. EGFR-independent mechanisms of acquired resistance to AZD9291 in EGFR T790M-positive NSCLC patients. Ann Oncol 2015;26:2073-8. [Crossref] [PubMed]
- Kim TM, Song A, Kim DW, et al. Mechanisms of Acquired Resistance to AZD9291: A Mutation-Selective, Irreversible EGFR Inhibitor. J Thorac Oncol 2015;10:1736-44. [Crossref] [PubMed]
- Uchibori K, Inase N, Araki M, et al. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat Commun 2017;8:14768. [Crossref] [PubMed]
- Jia Y, Yun CH, Park E, et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature 2016;534:129-32. [Crossref] [PubMed]
- Oxnard GR, Thress KS, Alden RS, et al. Association Between Plasma Genotyping and Outcomes of Treatment With Osimertinib (AZD9291) in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:3375-82. [Crossref] [PubMed]
- Sacher AG, Paweletz C, Dahlberg SE, et al. Prospective Validation of Rapid Plasma Genotyping for the Detection of EGFR and KRAS Mutations in Advanced Lung Cancer. JAMA Oncol 2016;2:1014-22. [Crossref] [PubMed]
- Sundaresan TK, Sequist LV, Heymach JV, et al. Detection of T790M, the Acquired Resistance EGFR Mutation, by Tumor Biopsy versus Noninvasive Blood-Based Analyses. Clin Cancer Res 2016;22:1103-10. [Crossref] [PubMed]
- Karlovich C, Goldman JW, Sun JM, et al. Assessment of EGFR Mutation Status in Matched Plasma and Tumor Tissue of NSCLC Patients from a Phase I Study of Rociletinib (CO-1686). Clin Cancer Res 2016;22:2386-95. [Crossref] [PubMed]
- Mok T, Wu YL, Lee JS, et al. Detection and Dynamic Changes of EGFR Mutations from Circulating Tumor DNA as a Predictor of Survival Outcomes in NSCLC Patients Treated with First-line Intercalated Erlotinib and Chemotherapy. Clin Cancer Res 2015;21:3196-203. [Crossref] [PubMed]
- Jiang T, Ren S, Zhou C. Role of circulating-tumor DNA analysis in non-small cell lung cancer. Lung Cancer 2015;90:128-34. [Crossref] [PubMed]
- Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol 2012;13:e23-31. [Crossref] [PubMed]
- Yasuda H, Park E, Yun CH, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med 2013;5. [Crossref] [PubMed]
- Oxnard GR, Lo PC, Nishino M, et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol 2013;8:179-84. [Crossref] [PubMed]
- Robichaux JP, Elamin YY, Tan Z, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med 2018;24:638-46. [Crossref] [PubMed]
- Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of Brain Metastases in Tyrosine Kinase Inhibitor-Naive Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. J Clin Oncol 2017;35:1070-7. [Crossref] [PubMed]
- Park KS, Raffeld M, Moon YW, et al. CRIPTO1 expression in EGFR-mutant NSCLC elicits intrinsic EGFR-inhibitor resistance. J Clin Invest 2014;124:3003-15. [Crossref] [PubMed]
- Lv C, An C, Feng Q, et al. A Retrospective Study of Stage I to IIIa Lung Adenocarcinoma After Resection: What Is the Optimal Adjuvant Modality for Patients With an EGFR Mutation? Clin Lung Cancer 2015;16:e173-81. [Crossref] [PubMed]
- Milovancev A, Stojsic V, Zaric B, et al. EGFR-TKIs in adjuvant treatment of lung cancer: to give or not to give? Onco Targets Ther 2015;8:2915-21. [PubMed]
- Huang Q, Li J, Sun Y, et al. Efficacy of EGFR Tyrosine Kinase Inhibitors in the Adjuvant Treatment for Operable Non-small Cell Lung Cancer by a Meta-Analysis. Chest 2016;149:1384-92. [Crossref] [PubMed]
- D'Angelo SP, Janjigian YY, Ahye N, et al. Distinct clinical course of EGFR-mutant resected lung cancers: results of testing of 1118 surgical specimens and effects of adjuvant gefitinib and erlotinib. J Thorac Oncol 2012;7:1815-22. [Crossref] [PubMed]
- Goss GD, O'Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 2013;31:3320-6. [Crossref] [PubMed]
- Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant Erlotinib Versus Placebo in Patients With Stage IB-IIIA Non-Small-Cell Lung Cancer (RADIANT): A Randomized, Double-Blind, Phase III Trial. J Clin Oncol 2015;33:4007-14. [Crossref] [PubMed]