Integrating primary care of chronic respiratory disease, cardiovascular disease and diabetes in Brazil: Practical Approach to Care Kit (PACK Brazil): study protocol for randomised controlled trials
Introduction
Chronic non-communicable diseases (NCDs) place a heavy burden on populations and health services in Brazil and other middle-income countries (1). In 2016 NCDs caused about 76% of all deaths in Brazil (2). While it is necessary to prevent these diseases by tackling causes such as smoking, diet, and physical inactivity, primary health care is important for identifying NCDs early and optimising management. The Global Alliance against Chronic Respiratory Diseases (GARD) aims to prevent and reduce the burden of chronic respiratory disease (CRD) in low, middle and high-income countries through multisectoral actions (3). GARD strategy complements global strategies to prevent and control other NCDs such as cardiovascular disease (CVD), diabetes and cancer (3). Since GARD was launched in Brazil in 2006 there have been several advances towards controlling CRD, including strengthening the capacity of doctors and nurses to manage CRD in primary care (4).
The prevalence of NCD multimorbidity in Brazil is high (5,6), and will keep increasing as the population ages. Multimorbidity challenges primary health care professionals to consider, diagnose and treat additional diseases in patients who are already being treated for other chronic conditions. Brazil’s Family Health Programme provides a good base for developing, evaluating and upscaling initiatives to address NCDs, as it is a primary care system providing free continuing care in health centres, staffed by doctors, nurses and other health professionals in municipalities through the country. Established in 1998, by 2010 it had expanded to cover 98 million people and 85% of Brazil’s municipalities (5). This paper reports on plans to evaluate the effectiveness of a new way of tackling these problems in Brazilian primary care.
Better primary care can improve chronic disease outcomes
CRD was identified as one of four priority disease areas by the UN General Assembly of 2012, which urged governments to provide all people with access to affordable care (7). Asthma and chronic obstructive pulmonary disease (COPD) account for most morbidity and mortality attributable to CRDs (8,9). Low and middle-income countries carry a heavy CRD burden, but can greatly reduce hospitalizations and deaths from asthma and COPD by improved access to care and appropriate treatment. The national database of the Ministry of Health of Brazil shows that, over 10 years, municipalities that provided free inhaled corticosteroids (ICSs) for asthma had considerably higher odds of reducing hospital admissions and deaths from asthma than those that did not (10). Programmes to improve asthma care in the Brazilian cities of Salvador and Londrina, which included physician education, and free ICSs and long acting beta2 agonists, resulted in large reductions in hospital admissions (11,12).
CVD is the leading cause of death in Brazil, even though age standardised death rates have decreased over the past 20 years (1). Expansion of the Family Health Programme was associated with about 20% reduction in mortality from heart and cerebrovascular disease from 2000 to 2009, showing the potential for primary care to reduce CVD burden (13). Although diabetes is less common, diabetes mortality has not decreased (1), and diabetes is often poorly controlled in Brazilian primary care (14). Diabetes often co-exists with other cardiovascular risk factors and disease, and integrated management of all CVD risks is needed. Hypertension is an especially important risk factor for diabetes, cardiovascular and cerebrovascular disease. As with CRD, these studies also show that good primary care can improve population health, but that integrated chronic disease management is still needed (10-12).
Implementing evidence-based chronic disease management
The Knowledge Translation Unit (KTU) of the University of Cape Town Lung Institute has been working since 2000 to strengthen primary health care in low and middle-income countries. It has developed several models for improving access to care, promoting early diagnosis and increasing access to evidence-based treatment for patients with priority conditions including common chronic diseases (15). To improve access to care, it has focussed on strengthening primary care services, encouraging evidence-based prescription policies and a clinical team approach, and, where possible and appropriate, task sharing with nurses, pharmacists and other staff. To improve capacity and quality of care it developed clinical decision support tools (CDST) and a training programme. Over-stretched clinicians often feel ill-equipped to deal with NCDs in primary care facilities, confused by often contradictory disease-specific guidelines. They can be empowered if provided with CDSTs that are localized to match their practice setting, scope of practice, resources and policies of the service in which they work, and to be evidence-based and up to date. KTU’s approach to training conforms to modern adult learning methods: interactive, case-based, and continuous, and offered by a trusted trainer on-site and in-service (16,17). This form of integrated care, using standardised diagnostic and treatment algorithms, is a recognized method for improving case detection, diagnosis and management, other examples being the WHO’s Integrated Management of Childhood Illness (IMCI) (18) and Package of Essential Non-Communicable Disease Interventions (WHO PEN) (19).
KTU first examined problems with diagnosis of asthma and COPD in South African primary care in the absence of routinely available spirometry (15,20). It then implemented a South African version of WHO’s Practical Approach to Lung Health (21), then expanded incrementally, to include sexually transmitted diseases and HIV infection (22), nurse-led antiretroviral treatment (23), and CVDs, diabetes, mental health and women’s health (24). Each version of the KTU tools has been evaluated in pragmatic randomized trials, in South Africa (21-24) and as a case study in Malawi (25). Over 250,000 copies of the CDSTs have been distributed in South Africa (where it is known currently as Adult Primary Care), and customized versions provided for Malawi, Botswana, Mexico City, Nigeria and Ethiopia. Globally, this programme is known as the Practical Approach to Care Kit (PACK) and is being spread through a non-profit partnership with the BMJ Publishing Group whose Knowledge Centre is responsible for developing more comprehensive clinical decision support mainly for high income country settings (26).
Development and implementation of PACK Adult in Brazil
PACK Adult in Brazil is the first localisation of PACK Global for adults led by an in-country team with mentorship provided by the KTU. Previously localisations outside of South Africa were either produced with intensive input from the KTU (Malawi, Botswana), or conducted independently (Mexico City). Work on PACK Brazil began in 2014 when the KTU entered an agreement with the City Health Department of Florianopolis, and BMJ Publishing Group to test a new mentorship model of spreading the PACK Global programme. Florianopolis is typical of a middle-income country urban setting with socioeconomic and health inequalities in which, despite good primary care coverage by official indicators, there are problems with accessibility to health care (only about half of the population use state-funded Family Health Programme clinics each year). Residents are registered with a local municipal primary care practice, which provides free medicines, while 44% of the population also has some health insurance. City Health Department doctors and other staff localised the PACK Adult content, ensuring compliance with Brazilian clinical protocols and practice, and translated it into Brazilian Portuguese. The training programme was based on South African PACK training, and revised with case studies and key messages localised to Brazilian priorities. The effectiveness of the training will be evaluated using the methods reported in this paper.
Methods
Objective
To determine the effectiveness of PACK Adult training with PACK CDST compared to passive dissemination of PACK CDST on the process of care and clinical outcomes for people with CRD, CVD or diabetes in primary care.
Hypothesis
PACK Adult training to use the CDST will increase investigations and diagnoses of CRD, CVD and diabetes; increase prescription of effective treatments; and improve health outcomes, compared to provision of a CDST without training.
Design
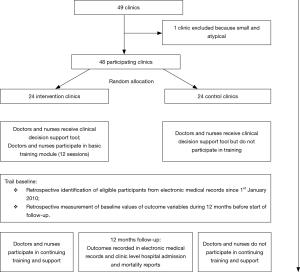
Pragmatic, parallel-group, superiority cluster randomised trial (27). All municipal clinics in Florianopolis were eligible for inclusion in the trial. However, 48 of all 49 municipal primary care clinics will be randomised to intervention or control groups (only one small clinic, with 600 registered patients, will be excluded so as to have equal numbers of clinics in each arm). Outcomes will be recorded at baseline and during 12 months’ follow-up (Figure 1).
Location
The trial will be conducted in the city of Florianopolis, Brazil.
Random allocation
Clinics will be stratified by size (numbers of patient attendances during 2015, and numbers of doctor-nurse teams) and socio-economic status of the communities they serve, and randomly allocated to intervention and control arms in a 1:1 ratio using N-Query Advisor by C Lombard before the intervention begins using a wait list control design. Training in control clinics will begin after follow up ends.
Blinding
Blinding of health professionals is not possible because of the nature of the intervention. Outcomes will be recorded without patient input so blinding of patient is not necessary. However, in practice patients will not be informed whether training had been delivered in their clinics or not.
Study populations
Eligible patients will be identified using International Classification of Disease (ICD-10) diagnostic codes in primary care electronic medical records (EMRs) of each clinic visit, in a consolidated municipal database. Eligibility criteria are as follows.
CRD trial
All patients aged 18 years and over in March 2017 who attend a clinic during the first year of the trial, with either a clinical diagnosis of obstructive lung disease (ICD-10 codes 40-47) recorded in EMRs since January 1st 2010 (when EMRs began, for estimation of asthma and COPD scores), or who attend a clinic for any reason during the year of follow-up (for estimation of rates or new asthma and COPD diagnosis).
CVD and diabetes trial
All patients aged 35 years and over in March 2017 and with a diagnosis of hypertension (ICD-10 I10-I15), ischaemic heart disease (ICD-10 I20-I25), heart failure (ICD-10 I50), cerebrovascular disease (ICD-10 I60-I69), or diabetes mellitus (ICD-10 E10-E14, that is, including type 1 and type 2 diabetes) ever recorded since January 1st 2010, and who attend a participating clinic for any reason during the first year of the trial.
For both trials, the first year of the trial is defined as 1 April 2017 to 31 March 2018, and the baseline year is defined as 1 April 2016 to 31 March 2017.
Intervention group clinics (28)
Primary care doctors and nurses will get outreach education and copies of the PACK Adult CDST. The CDST is a printed book which is also available in electronic form. Staff training will comprise educational outreach sessions, using local practitioners (doctors and nurses) as trainers. Educational outreach training sessions are brief personal visits to health professionals at their place of practice. The training will use a cascade model, with four master trainers training and supporting twenty facility trainers who will then deliver the on-site sessions to the 24 intervention clinics. The facility trainers will be professionals recognised as clinical leaders by the staff and work as clinical mentors, enhancing knowledge exchange between staff and facilitating implementation of the PACK strategy. They will work in doctor/nurse pairs to train small doctor/nurse groups in the facility during 12 weekly sessions covering the first module of guideline content—including CRDs, CVD, and diabetes. The focus of the training is the approach to using the CDST in managing a variety of patients, and highlights key messages about the identification of the conditions, their appropriate management and secondary level referral. After an initial intensive overview of CDST and how to use it, the rest of the CDST content will be covered in monthly on-site sessions. Continual clinical support will be provided by the family physician who led the localisation of the CDST (R Zonta). Electronic discussions will be posted on a web discussion site and accessed by all trainers and trainees. Individuals’ participation in training sessions will be monitored to assess intervention coverage.
Control group clinics
Primary care doctors and nurses will get copies of the PACK CDST, without training, assuming that passive dissemination of printed materials alone have negligible effects on clinician behaviour (29).
Trial outcomes
The outcomes include indicators of health status, and of health care processes prioritised in the CDST and training and involving treatments and tests that are available in the trial clinics. Because the needs of different patients differ, it is not possible to assess appropriateness of interventions and treatment in individual patients. Rather, within each category of disease, we have selected processes which, when applied to groups of patients, result in favourable health outcomes. The composite outcomes reflect a variety of appropriate clinical responses for that category of patient. Thus, we will be able to detect changes and differences in the frequency of these selected composite process outcomes in the study population.
CRD trial
Primary outcomes
The three primary outcomes selected for this study represent key health care processes that have been shown in Brazil and other countries to be associated with better health outcomes in patients with asthma and COPD, reflect the quality and intensity of respiratory care, and are based on actions recommended in the CDST (30,31). The use of composite scores as primary outcomes is similar to WHO’s Multi Country Evaluation of IMCI (32,33).
- For patients with asthma the composite score will comprise points awarded for: (I) a first prescription of an ICS or ICS+ long-acting bronchodilator [long-acting beta2 agonist (LABA)] combination, or a change in prescription, stepping up from short-acting bronchodilator [short-acting beta2 agonist (SABA)] to ICS or from ICS to long-acting bronchodilator (LABA) + ICS combination; or stepping down from LABA + ICS to ICS, or from ICS to SABA (scoring one point if at least one of these occurs); and (II) request for spirometry (1 point). The composite score will be the sum of these points, and will thus range from 0 to 2;
- For patients with COPD the composite score will comprise points awarded for: (I) a first prescription of SABA, ICS, or ICS + LABA; or a change in prescription, stepping up from SABA to LABA or LABA to ICS + LABA, or stepping down from LABA + ICS to LABA, or from LABA to SABA (scoring one point if at least one of these occurs) and (II) request for spirometry (1 point). The composite score will be the sum of these, and will thus also range from 0 to 2;
- At clinic level, the incidence rate of new diagnoses of asthma or COPD among all patients who use each clinic for the duration of the trial. This endpoint addresses the problem of under-diagnosis of these conditions which the CDST aims to address.
Secondary outcomes
At clinic level: hospital admission rate for asthma in each clinic; hospital admission rate for COPD in each clinic and, at patient level: CVD disease (ICD-10 I00-I99) diagnosed for the first time; diabetes mellitus (ICD-10 E10-E14) diagnosed for the first time; medications to support tobacco cessation (nicotine replacement therapy, nortriptyline, or bupropion prescribed); blood pressure recorded, or cholesterol, glucose, or electrocardiogram tests recorded; depression (ICD-10 F32-F34) diagnosed for the first time; medication for depression (tricyclic and related antidepressants, selective serotonin re-uptake inhibitors, and monoamine oxidase inhibitors) prescribed for the first time; death from any cause and from respiratory disease. The outcomes relating to CVD, diabetes and depression indicate increased awareness and management of comorbidity in patients with CRD.
CVD and diabetes trial
Primary outcomes
The two primary outcomes indicate assessment of cardiovascular risk and comorbidity, and control of blood pressure which is relevant to all CVD and diabetes.
- Number of participants in whom at least one of the following tests was recorded: body mass index, plasma glucose, glycated haemoglobin (HbA1c), serum cholesterol, electrocardiogram;
- In participants with systolic blood pressure (SBP) >140 mmHg recorded during 12 months before start of follow up, average SBP recorded.
Secondary outcomes
These outcomes are indicators of more active clinical management or health outcome. Outcomes relating to depression indicate increased awareness and management of comorbid depression. Outcomes measured at patient level will be: number of tests for cardiovascular and diabetes risk factors (body mass index, glucose tested, serum cholesterol tested, electrocardiogram, chest X-ray); mean diastolic blood pressure (DBP); statin prescribed for the first time; statin dose changed; depression diagnosed for the first time; antidepressant prescribed; heart failure diagnosed for the first time; ischemic heart disease diagnosed for the first time; cerebrovascular disease diagnosed for the first time; in participants with a diagnosis of heart failure, new prescriptions for diuretic, angiotensin-converting enzyme (ACE) inhibitor or beta blocker; in participants with a diagnosis of hypertension, prescription for increased dose of diuretic, ACE inhibitor, calcium channel blocker or beta blocker; in participants with a diagnosis of ischemic heart disease, new prescription of ACE inhibitor, nitrate, beta blocker, ACE inhibitor or angiotensin II receptor antagonist; in participants with diabetes, change in prescription from oral diabetes medication to insulin; specialist referral to pulmonology, cardiology or endocrinology; in participants with SBP >140 mmHg or DBP >90 mmHg recorded during 12 months before enrolment, SBP ≤140 mmHg and DBP ≤90 mmHg. Outcomes measured at clinic level will be: hospital admission rates for CVD or diabetes; rate of death from any cause and from CVD or diabetes.
Data collection and management
Quantitative data on outcomes and baseline variables will be extracted from the municipal health department’s EMRs which include every clinic. Clinical data (including coded symptoms, ICD-10 coded diagnoses, prescriptions and test requests) are entered during each consultation by a doctor or nurse, and linked at city, practice and patient levels. The database is actively managed and regularly interrogated by the lead doctor (MP de Andrade). Aggregate hospital admission and mortality data are recorded at clinic level and cannot yet be linked to individual patients; they will be monitored as indicators of potential adverse effects of the intervention. A data monitoring committee was not needed because the trial used only routinely collected EMRs and aggregated data which the investigators had critically evaluated for the 7 years before follow-up began. The principal investigators and City Health Department doctors will have access to the data.
Sample size and power
CRD trial
The following estimates are based on analysis of EMRs from participating clinics in 2016. Numbers eventually recruited into the trial may differ as they will depend on clinic visits and diagnoses recorded during the baseline year and year of follow-up. About 2,900 eligible patients with a diagnosis of asthma and 1,400 with COPD were recorded as visiting participating clinics during 2016. That is, in each clinic there will be an average of about 60 eligible patients with asthma and 28 with COPD. The mean outcome scores per patient were 0.43 [SD 0.50, intracluster correlation coefficient (ICC) 0.033] for asthma and 0.42 (SD 0.48, ICC 0.055) for COPD. The trial will therefore have 90% power to detect a 26% increase in mean asthma score (0.54 vs. 0.43), and a 33% increase in mean COPD score (0.56 vs. 0.42), with 5% significance. At clinic level, the mean annual rate of new diagnoses or asthma or COPD was 1.1 (SD 0.46) per 100 patients aged ≥18 attending for any reason, providing 85% power to detect a 36% increase in rates of diagnosis (1.5 vs. 1.1 per 100/year). With Bonferroni correction to the significance level, to account for having three primary outcomes (P=0.05/3=0.017), the power to detect these differences as significant is 84%, 82% and 73%, respectively.
CVD and diabetes trial
There were 15,600 eligible patients with SBP >140 mmHg recorded in 2016, that is, an average of about 325 eligible patients with the primary outcome recorded in each clinic. In 2016, mean SBP in these patients was 138 (SD 17) mmHg, ICC =0.015. This provides 96% power to detect a difference in mean SBP of 2.5 mmHg or more between trial arms, with 5% significance. Of about 28,550 eligible patients (595 per clinic), 49% had glucose, cholesterol, ECG or chest X-ray tested during 2016, ICC =0.069. This provides 92% power to detect a 12% difference (49% vs. 61%) in the proportion who have at least one of these tests, with 5% significance. With a 2.5% significance level to account for having two primary outcomes (P=0.05/2=0.025), for each endpoint the power to detect these differences as significant is 93% and 87%, respectively.
Statistical analysis
We will compare primary and secondary trial outcomes between intervention and control clinics, using multilevel random effect regression models to adjust for the cluster randomised design in individual level analyses, and for baseline covariates if they are unbalanced. Analyses of asthma and COPD scores and SBP, as primary outcomes, will be at individual participant level. Analyses of rates of asthma and COPD diagnosis, hospital admission and mortality will be at clinic level. All other analyses of secondary outcomes will be at individual level. Analysis will be by intention to treat. Analyses of SBP as primary outcome will use the mean of all SBPs recorded during 12 months of follow-up, and baseline covariates will include mean SBP recorded during 12 months before the start of follow-up. Subgroup analyses will investigate whether intervention effects are modified by clinic size, doctor-nurse ratio, gender, age, disease and area deprivation level, by adding interaction variables to the regression models. Secondary analyses will explore potential time trends in effects within the year of follow-up, and also for 12 months after that year (when control clinics will receive the intervention). There will be no interim analyses or stopping rules.
Ethics and research governance
Ethical guidance on cluster randomized trials and on use of medical records for research will be followed, as follows (34,35). Professionals and managers in each clinic have consented to their clinic taking part in the trial. The trials will be based on EMRs without patient contact and obtaining consent from each individual will not be feasible. It is not feasible to obtain patients’ consent to be randomised to intervention or control arms, because randomisation and delivery of the intervention will be at clinic level. Intervention- and control-type care cannot be provided to different patients within the same clinic because of the practicalities of clinic staffing, training and management (28). Even if patients preferred doctor- or nurse-led care or did not consent to take part in the trial, they would thus still necessarily receive the type of care that the clinic was allocated to provide.
Patients will not be asked for consent for their EMRs to be used for this research because it is not feasible without greatly increasing research costs. However, we will adhere to the ethical principles for use of medical records without patients’ consent (29), as follows. The research has a clear public benefit. We have obtained approval for the study from the lead doctors and nurses managing the programme. Use of the data for research will not influence decisions about individuals’ care. Only health department data managers have access to personal identifiers. Data with patient identifiers will be held by the Florianopolis City Health Department, and anonymised unlinked data will be securely stored at the University of East Anglia and the University of Cape Town.
Discussion
These randomised trials will evaluate the effects of a complex educational and health systems intervention aimed at primary care professionals and intended to improve clinical practice and health outcomes among adults with respiratory disease, CVD or diabetes. The trial focuses in particular on diagnosis of neglected and comorbid conditions, on initiation and modification of treatment, and blood pressure control which is clinically important for all CVDs and diabetes. Increased provision of preventive treatment for CRD, and better management of vascular risk in people with CVDs and diabetes, are well recognised as priorities for middle income countries like Brazil. However, achieving these aims can be difficult. Several trials have attempted to improve the management of multimorbidity in primary care in high income countries, with mixed results (36), and others are under way (37-40), but evidence from middle income countries is lacking. It will be valuable to know whether this type of intervention, which has been shown to be effective in South Africa, will be effective in Brazil.
Randomised trials of complex interventions in real-world health systems to address several diseases together face methodological challenges. Specifying one primary outcome may be inappropriate if one is equally concerned about more than one health condition, and if one aims to change several aspects of professional practice. Professional educational interventions covering a range of conditions delivered to many facilities and to diverse professionals as part of their routine practice may have small effects, some of which may take years to improve health outcomes. To overcome these challenges, such trials may need large samples, long follow-up and major research funding, which can be difficult to raise in settings where they are most needed.
This protocol aims to address these challenges. It proposes two simultaneous trials, with each covering a group of closely related conditions, having more than one primary outcome and with adequate statistical power. It is noteworthy that lowering the statistical significance level to account for more than one primary outcome does not greatly reduce power. We are able to obtain large samples, especially for the CVD and diabetes trial, by including all eligible municipal clinic users in a medium-sized city, using EMRs for enrolment and outcome measurement. In addition to assessing the biological effects of the intervention on blood pressure, which is highly important to all of the cardiovascular conditions and diabetes, the trials will be able assess evidence of intensification of investigation and treatment of these important but often neglected conditions. Inclusion of a whole Brazilian city enhances the generalisability of the trials.
The proposed trials have limitations, partly due to the limitations of the EMR data and of other resources. Although they have good quality data on diagnoses, investigations, prescriptions and referrals, which accurately indicate clinical practice, the EMRs have limited data on health outcomes at individual patient level. Relevant outcomes such as symptom severity, blood chemistry, lung function, and quality of life are not available. However, clinic level disease-specific rates of hospital admissions and deaths are recorded and are included in the trials’ secondary outcomes. Although the cluster randomisation design was intended to avoid contamination between intervention and control clinics, contamination is still possible, for example by patients switching between clinics, by staff transfers between clinics, and by communication between professionals working in intervention and control clinics. We will analyse the EMRs to assess the risk of contamination by patient switches and staff transfers. Lastly, although the study population includes all public sector primary clinics in the city, it excludes private, specialist and inpatient care. Inclusion of these services would require a much more complex intervention which was not feasible with available resources and local support.
In summary, routine use of EMRs is increasing rapidly in Brazilian primary care, and this protocol shows how they could be used more widely to evaluate system-wide interventions. If shown to be effective, this approach to improving the quality of care could be implemented more widely in Brazil and in other low and middle-income countries. The most direct relevance, however, will be towards finding practical ways of improving clinical management and health outcomes of adults with respiratory and CVD and diabetes in Brazilian public sector primary care.
Acknowledgements
The authors are grateful for the Florianopolis City Health Department for permission to carry out the study and for funding the intervention, to the doctors and nurses in intervention clinics who participate in the training and using of the CDST; to Dr. Tracy Eastman (Director of PACK Global Development at KTU/BMJ Publishing) for support with agreements, project management and quality assurance; and to the Peter Sowerby Foundation for financial support for the Brazilian localisation of PACK Adult. This work was supported by the Florianopolis City Health Department which funded in-country localisation of PACK Adult, printing of CDST, training and clinical support are funded by. Use of PACK Adult CDST and associated materials is provided free of charge to Florianopolis City Health Department by BMJ Publishing on behalf of the KTU. Mentoring of the localisation process, including development of tools and materials to support that process, by the KTU was funded by the Peter Sowerby Foundation (http://www.petersowerbyfoundation.com/).
Footnote
Conflicts of Interest: The Florianopolis City Health Department has a non-financial interest, and the KTU and BMJ Publishing have a financial interest, in the intervention being shown to be effective. The KTU and BMJ Publishing have entered into a non-profit partnership to explore ways of making annual revisions of the PACK CDST and associated materials globally available, and to provide mentorship support to in-country teams wishing to localise it for their settings. The authors declare that they have no other competing interests.
Ethical Statement: The protocol was approved by the Research Ethics Committee of the Federal University of Santa Catarina (approval number: 1.539.125). Permission to carry out the trial was provided by the Florianopolis City Health Department. Patients will not be asked to provide consent to participate, for the reasons discussed in Ethics and research governance. Participation in the study will not affect patients’ future management.
References
- Schmidt MI, Duncan BB, Silva GA, et al. Chronic non-communicable diseases in Brazil: burden and current challenges. Lancet 2011;377:1949-61. [Crossref] [PubMed]
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1151-210. [Crossref] [PubMed]
- Khaltaev N. GARD, a new way to battle with chronic respiratory diseases, from disease oriented programmes to global partnership. J Thorac Dis 2017;9:4676-89. [Crossref] [PubMed]
- Cruz AA, Camargos PA, Urrutia-Pereira M, et al. Global Alliance against Chronic Respiratory Diseases (GARD) Brazil success case: overcoming barriers. J Thorac Dis 2018;10:534-8. [Crossref] [PubMed]
- Rzewuska M, de Azevedo-Marques JM, Coxon D, et al. Epidemiology of multimorbidity within the Brazilian adult general population: Evidence from the 2013 National Health Survey (PNS 2013). PLoS One 2017;12. [Crossref] [PubMed]
- Paim J, Travassos C, Almeida C, et al. The Brazilian health system. Lancet 2011;377:1778-97. [Crossref] [PubMed]
- UN General Assembly. Draft Resolution A/67/L.36. Global Health and Foreign Policy 2012. Accessed 12 July 2018. Available online: https://documents-dds-ny.un.org/doc/UNDOC/LTD/N12/630/51/PDF/N1263051.pdf?OpenElement
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic review. Lancet 2012;380:2095-128. [Crossref] [PubMed]
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries. Lancet 2012;380:2163-96. [Crossref] [PubMed]
- Ponte EV, Cruz AA, Athanazio R, et al. Urbanization is associated with increased asthma morbidity and mortality in Brazil. Clin Respir J 2018;12:410-7. [Crossref] [PubMed]
- Cerci Neto A, Ferreira Filho OF, Bueno T, et al. Reduction in the number of asthma-related hospital admissions after the implementation of a multidisciplinary asthma control program in the city of Londrina, Brazil. J Bras Pneumol 2008;34:639-45. [Crossref] [PubMed]
- Souza-Machado C, Souza-Machado A, Franco R, et al. Rapid reduction in hospitalisations after an intervention to manage severe asthma. Eur Respir J 2010;35:515-21. [Crossref] [PubMed]
- Rasella D, Harhay MO, Pamponet ML, et al. Impact of primary health care on mortality from heart and cerebrovascular diseases in Brazil: a nationwide analysis of longitudinal data. BMJ 2014;349:g4014. [Crossref] [PubMed]
- Viana LV, Leitao CB, Kramer CK, et al. Poor glycaemic control in Brazilian patients with type 2 diabetes attending the public healthcare system: a cross-sectional study. BMJ Open 2013;3. [Crossref] [PubMed]
- English RG, Bateman ED, Zwarenstein MF, et al. Development of a South African integrated syndromic respiratory disease guideline for primary care. Prim Care Respir J 2008;17:156-63. [Crossref] [PubMed]
- Stein J, Lewin S, Fairall L, et al. Building capacity for antiretroviral delivery in South Africa: A qualitative evaluation of the PALSA PLUS nurse training programme. BMC Health Serv Res 2008;8:240. [Crossref] [PubMed]
- Fairall L, Bateman E, Cornick R, et al. Innovating to improve primary care in less developed countries: towards a global model. BMJ Innov 2015;1:196-203. [Crossref] [PubMed]
- World Health Organisation. IMCI Chart Booklet. 2014. Accessed 11 June 2018. Available online: http://who.int/maternal_child_adolescent/documents/IMCI_chartbooklet/en/
- World Health Organisation. Prevention and control of noncommunicable diseases: guidelines for primary health care in low resource settings. 2012. Accessed 11 June 2018. Available online: http://apps.who.int/iris/bitstream/10665/76173/1/9789241548397_eng.pdf
- English RG, Bachmann MO, Bateman ED, et al. Diagnostic accuracy of an integrated respiratory guideline in identifying patients with respiratory symptoms requiring screening for pulmonary tuberculosis: a cross-sectional study. BMC Pulm Med 2006;6:22. [Crossref] [PubMed]
- Fairall LR, Zwarenstein M, Bateman ED, et al. Effect of educational outreach to nurses on tuberculosis case detection and primary care of respiratory illness: pragmatic cluster randomised controlled trial. BMJ 2005;331:750-4. [Crossref] [PubMed]
- Zwarenstein M, Fairall LR, Lombard C, et al. Outreach education integrates HIV/AIDS/ART in South African primary care clinics: a pragmatic cluster randomized trial. BMJ 2011;21:342:d2022.
- Fairall L, Bachmann MO, Lombard C, et al. Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): a pragmatic, parallel, cluster-randomised trial. Lancet 2012;380:889-98. [Crossref] [PubMed]
- Fairall LR, Folb N, Timmerman V, et al. Educational outreach in an integrated clinical management tool for nurse-led non-communicable chronic disease management in primary care in South Africa: pragmatic cluster randomised controlled trial. PLOS Med 2016;13. [Crossref] [PubMed]
- Schull MJ, Cornick R, Thompson S, et al. From PALSA PLUS to PALM PLUS: adapting and developing a South African guideline and training intervention to better integrate HIV/AIDS care with primary care in rural health centers in Malawi. Implement Sci 2011;6:82. [Crossref] [PubMed]
- Knowledge Translation Unit. PACK Global Adult 2017 eBook. 2017. Accessed 11 June 2018. Available online: http://pack.bmj.com/pack-global-adult-2016-ebook-v1/
- Campbell MK, Piaggio G, Elbourne DR, et al. CONSORT 2010 statement: extension to cluster randomised trials. BMJ 2012;345. [Crossref] [PubMed]
- Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [Crossref] [PubMed]
- Giguère A, Légaré F, Grimshaw J, et al. Printed educational materials: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2012;10. [PubMed]
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention 2018. Accessed 11 June 2018. Available online: http://ginasthma.org/
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Am J Respir Crit Care Med 2017;195:557-82. [Crossref] [PubMed]
- Huicho L, Scerpbier RW, Nkowane AM, et al. How much does quality of child care vary between health workers with differing durations of training? Lancet 2008;372:910-16. [Crossref] [PubMed]
- Gouws E, Bryce J, Pariyo G, et al. Measuring the quality of child health care at first-level facilities. Soc Sci Med 2005;61:613-25. [Crossref] [PubMed]
- Medical Research Council. Cluster Randomised Trials – Methodological and Ethical Considerations. 2002. Accessed 11 June 2018. Available online: https://www.cebma.org/wp-content/uploads/Cluster-randomised-trials-Methodological-and-ethical-considerations.pdf
- Haines A, Ashcroft R, Coggon D, et al. Personal Information in Medical Research. 2000. Accessed 11 June 2018. Available online: https://www.mrc.ac.uk/documents/pdf/personal-information-in-medical-research/
- Smith SM, Soubhi H, Fortin M, et al. Managing patients with multimorbidity: systematic review of interventions in primary care and community settings. BMJ 2012;345. [Crossref] [PubMed]
- Healey EL, Jinks C, Tan VA, et al. Improving the care of people with long-term conditions in primary care: protocol for the ENHANCE pilot trial. J Comorb 2015;5:135-49. [Crossref] [PubMed]
- Man MS, Chaplin K, Mann C, et al. Improving the management of multimorbidity in general practice: protocol of a cluster randomised controlled trial (The 3D Study). BMJ Open 2016;6. [Crossref] [PubMed]
- Liddy C, Hogg W, Singh J, et al. A real-world stepped wedge cluster randomized trial of practice facilitation to improve cardiovascular care. Implement Sci 2015;10:150. [Crossref] [PubMed]
- Bozorgmehr K, Szecsenyi J, Ose D, et al. Practice network-based care management for patients with type 2 diabetes and multiple comorbidities (GEDIMAplus): study protocol for a randomized controlled trial. Trials 2014;15:243. [Crossref] [PubMed]