Lung donor selection criteria
Introduction
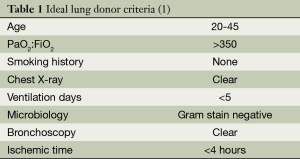
Lung transplantation is an established therapy for selected patients with end-stage pulmonary disease. Since the first successful lung transplant in 1983 by Dr. Joel Cooper and his team, over 42,000 recipients have benefitted from this procedure worldwide. Advances in surgical techniques, postoperative care, and immunosuppression therapy have led to improved short- and long-term survival following lung transplantation. Despite this success, the number of suitable lung donors remains a significant limitation. Today many donors are judged based on empiric criteria developed in the 1980s (See Table 1) (2,3).
Most centers agree that these criteria are too strict and use extended criteria donors (ECD) that do not completely meet the traditional empiric criteria (4). Many centers advocate use of ECD to effectively increase the donor pool with similar transplant outcomes (2,5-10). There is considerable variation in practice patterns among these centers and no uniformly accepted discriminating metric (6).
In-hospital mortality for lung transplantation is higher than for other solid organs. A significant contributor to this early hazard is primary graft dysfunction (PGD) (11). PGD occurs in up to 25% of recipients with associated 30 days mortality of 40-50%; compared to 5-10% without PGD (12). Accumulating evidence suggests that PGD is the end result of a series of injuries occurring in the donor lung from the time of brain death to reperfusion in the recipient (13). Therefore, concern over PGD may drive concern over lung donors, and thus limit the number of organs considered usable for transplant. Given the increasing burden of lung disease, the extremely limited number of suitable lung donors, and increasing waitlist mortality, it is not surprising that an increasing numbers of ECDs are being used. In the era of the lung allocation score, with preferential allocation to sicker recipients, it becomes more important to understand not only which ideal criteria can be ignored, but also in which context. Here, we break down donor criteria by individual factors and examine their effect on outcomes.
Age
Over the last 30 years, the average age of donors accepted for transplant has steadily increased. Retrospective cohort analysis of OPTN data revealed no increases in one year graft failure with donors aged 18-64. Ages <18 and >64 were associated with increased failure rates at one year but were not associated with increased PGD (14). Retrospective review of UNOS data from 2000-2010 confirms an increase in 1- and 3-year mortality for donors over the age of 65 without increases in bronchiolitis obliterans syndrome (BOS) (15). Further stratification into age groups [50-54, 55-59 and 60-64] did not reveal differences in one year mortality or FEV1 (16). Available literature favors consistent outcomes for donors within the range of 18-64 years.
Gender
Donor and recipient gender combinations have been analyzed with mixed results. Fessart et al. failed to discern a difference in recipient survival after analysis of all gender combinations (17). Another single center retrospective study demonstrated an increase in survival and decrease in BOS for donor recipient gender mismatches (M→F and F→M). Male donor to male recipients specifically had a significant decrease in survival (18). International Society of Heart and Lung Transplant (ISHLT) registry review from 1995-2002 reflected a decreased survival in female donors to male recipients. Female donor to female recipient demonstrated a short and long term survival benefit (19). These results coincided with a multicenter study in France (20). The exact gender interactions between donor and recipient have yet to be defined to accurately shape our practice of transplant selection. There are questionable effects of hormones and size mismatch that have yet to be delineated in the literature.
Race
Retrospective review of lung transplants from 1997 to 2007 of race matched donors and recipients conferred a 3.3% decreased risk adjusted mortality at five years and 12% overall mortality in recipients with cystic fibrosis (CF), idiopathic pulmonary fibrosis (IPF) and single lung transplant (SLT). No changes in one year rejection rates were associated with race matching. Donor African American lungs reflected an increased risk of death regardless of recipient. Overall, specific recipient race was not associated with survival variability (21).
Smoking history
In the UK, a smoking history in donor lungs is associated with decreased recipient survival as compared to non-smoker donor lungs. The recipient survival, however, remains greater than that of the wait list population (22). This raises the argument that patients with high mortality risk would benefit from transplantation rather than succumb to illness on the waiting list. The interpretation of this data is also limited given recipients of smoker lungs were riskier candidates prior to surgery. Smoker donor lungs confer a higher risk of grade 3 PGD (23). A retrospective review of UNOS data on 766 heavy smoker donor lungs (>20 pack year history) revealed no increases in BOS or median survival (24). An additional single retrospective study of smoking donors revealed a worse early survival but no effect on long term survival and BOS incidence (25). This was confirmed by an additional retrospective single institution study that had prolonged postoperative intubation and ICU stay in smokers but equivalent survival at three years (26). The overall findings coincide with an initial higher postoperative risk, and equivalent to higher long term recipient mortality risk, for smoker donor lungs as compared to non-smoker donor lungs. The mortality of patients receiving smoker donor lungs does reflect a lower mortality risk than that of patients on the transplant waiting list.
Bronchoscopic findings and cultures
Post transplantation pneumonia and sepsis are serious concerns to the transplant surgeon and previous guidelines for chest X-ray and bronchoscopy attempt to avoid transmission to immunosuppressed recipients. Gram stain evaluation of airways in a single center retrospective study found 12% of donors with a positive gram stain subsequently developed recipient pneumonia while 20% of negative gram stain donors went on to develop pneumonia. This refutes the association of donor gram stain with recipient pneumonia. In this study, however, donor lungs were not accepted if there was evidence of frank aspiration on bronchoscopy (27). Prospective analysis of donor airway cultures and bronchial tissue cultures revealed a <1.5% transmission rate of donor organ contamination (28). The lack of infection transmission from donor to non-suppurative based recipients is also been confirmed by two separate studies (29,30). With appropriate antibiotic prophylaxis to cover Pseudomonas and Staph aureus, risk of transmission of donor associated infection is negligible.
Radiographic findings
Donors undergo multiple radiographs prior to surgery. The high degree of interpretation variability have diminished the role in donor selection criteria (31). One third of possible donor radiographs in a retrospective survey had infiltrates, of which greater than half improved or spontaneously resolved. Improvement in infiltrates did not impact transplantation rates and led to unnecessary rejection. All patients transplanted in this study with positive infiltrates were alive at one year follow-up (32). No studies were found that correlated chest radiograph findings to recipient infections. The literature on radiographic donor exclusion is extremely limited, and the topic warrants further investigation.
Size mismatch
A recent review by Barnard published in 2013 thoroughly outlines size criteria for donor/recipient, and their results are briefly summarized here (33). Total lung capacity (TLC), recipient pathology (obstructive vs. restrictive), and height all factor in to appropriate matches. For double lung transplants, patients with emphysema should be matched to a donor with a 67-100% of the recipient’s TLC. No definitive data is available for SLT for emphysema. For pulmonary hypertension and CF patients, the predicted total lung capacity (pTLC) of the donor may safely reach 120% of the recipient actual TLC. Due to the limitations in TLC that occur in pulmonary fibrosis, the recommendation for donors pTLC is to be within 20% of the halfway point between the recipients actual TLC and pTLC. For SLT for fibrotics, the donor pTLC should be within 20% of the recipient’s pTLC. Little data exists for transplantation in overt size mismatch, but some suggest it is preferable to slightly oversize if possible and not undersize less than 80% (34).
Ischemic time and donor distance
Retrospective review of UNOS data of 6,055 transplants revealed no increased incidence of BOS or three years mortality in recipients with local, regional or national lung donors despite national ischemic times of (342±90) minutes (35). Additional single center studies verify no change in survival for ischemia greater than six hours (36-40). Donor ischemia time >7 hours and donor age >50 years compounded, however, was associated with decreased recipient survival at two years (41).
Donation after cardiac death
After evaluating the literature for effects of ischemia on recipient outcomes, the question of donation after cardiac death (DCD) use as opposed to beating heart brain dead donors inevitably follows. The largest single center study with 409 DCD lungs revealed a decrease in graft survival that did not reach statistical significance. The patient survival and BOS were comparable (42). Smaller, single center studies reveal either similar survival rates (43,44), or a modest decrement in survival (45). A single institutional study out of Madrid revealed PGD in 72%, Survival rates of 51% at five years, and BOS of 45% at five years (46). Use of DCD donor lungs revealed a 100% survival at almost a year in eight patients (47). In total, these studies suggest the benefit of using DCD donors as a means to expand the available donor pool.
High risk donors
The Centers for Disease Control and Prevention (CDC) label high risk donors as those with exposure to HIV, prison inmates, IV drug users, prostitution history, high risk sexual history, and hemophiliacs. Limited data is available for lung transplantation in CDC high risk donors. Review of UNOS database on CDC high risk donors demonstrated equivalent one year mortality, postoperative infection, stroke and dialysis with normal donors. Around 9% of lung donors were classified as high risk and risk of disease transmission was less than 1%. Interestingly 95% of recipients surveyed would accept an organ from a high risk donor with an expected donor pool expansion of 10% (48).
Oxygenation
Arterial partial pressure of oxygen (PaO2) is a traditional way to measure lung function. Donors with initial PaO2/FiO2 of <300, that improved to >300 with recruitment maneuvers, used in Australia were not associated with a decreased 30 days, 1, 2, 3 yrs survival or recipient PaO2/FiO2 ratio (8). High dose steroid administration after brain death was associated with an increase in PaO2/FiO2 of 16 +/-14 and a decrease of 34.2 +/-14 if steroids were not given. The outcome of recipients receiving steroid treated donor lungs was not analyzed in this study (49). Most importantly, UNOS data from 2000 to 2009 of 12,045 transplants failed to demonstrate a PaO2 association with decreased survival, even with a PaO2 of less than 200 in 1,830 patients (50). This may be due to preoperative gasses that are lower on initial reported PaO2 and significantly improve after recruitment maneuvers, which are not consistently captured in the database.
Ex vivo lung perfusion (EVLP)
EVLP is an emerging technique used to evaluate and potentially salvage high-risk donor organs typically not suitable for lung transplantation (51). Steen initially utilized this technique to evaluate a DCD donor (52) and their success has sparked several studies around the world (51,53-57). These studies have demonstrated similar length of mechanical ventilation, rate of PGD, length of stay and mortality. How this technology will be implemented in allocation has yet to be determined despite the considerable promise they imply. Despite these challenges, it appears that the future of lung transplantation will capitalize on EVLP to safely expand the donor pool by expanding the limits of what defines a suitable donor.
Conclusions
There is little data to suggest that any of the historical criteria for defining the ideal lung transplant donor impact either short or long term outcomes. For age, donors should be within 18 to 64 years old. Gender may relay benefit to all female recipients especially in male to female transplants. Negative outcomes are associated with female donors to male recipients. Race matched donor/recipients have improved outcomes and African American donors convey worse prognosis. Smoking donors may decrease recipient survival post transplant, but provide a life saving opportunity for recipients that may otherwise remain on the transplant waiting list. No specific gram stain or bronchoscopic findings are reflected in recipient outcomes. Chest radiographs are a poor indicator of lung donor function and should not adversely affect organ usage aside for concerns over malignancy. Ischemic time greater than six hours has no documented adverse effects on recipient mortality and should not limit donor retrieval distances. Brain dead donors and deceased donors have equivalent prognosis. Initial PaO2/FiO2 ratios less than 300 should not dissuade donor organ usage, although recruitment techniques should be implemented with intent to transplant.
Although there have been multiple trials on individual lung donor criteria that fail to show negative recipient prognosis (58), there are few studies that evaluate the effects of multiple extended criteria compounded together in one donor lung. These compromises in physiology may have untold effects on PGD and overall patient mortality. In additional to donor selection, it is imperative to consider the recipient’s pathology as a major harbinger of overall transplantation outcome (59). It is currently our recommendation that any single criteria outside of the historical ideals can safely be ignored, but we caution that the cumulative effects of multiple extended donation criteria in one donor have not been studied.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Botha P, Rostron AJ, Fisher AJ, et al. Current strategies in donor selection and management. Semin Thorac Cardiovasc Surg 2008;20:143-51. [PubMed]
- Filosso PL, Turello D, Cavallo A, et al. Lung donors selection criteria: a review. J Cardiovasc Surg (Torino) 2006;47:361-6. [PubMed]
- Bhorade SM, Vigneswaran W, McCabe MA, et al. Liberalization of donor criteria may expand the donor pool without adverse consequence in lung transplantation. J Heart Lung Transplant 2000;19:1199-204. [PubMed]
- Reyes KG, Mason DP, Thuita L, et al. Guidelines for donor lung selection: time for revision? Ann Thorac Surg 2010;89:1756-64; discussion 1764-5.
- Botha P, Trivedi D, Weir CJ, et al. Extended donor criteria in lung transplantation: impact on organ allocation. J Thorac Cardiovasc Surg 2006;131:1154-60. [PubMed]
- Meers C, Van Raemdonck D, Verleden GM, et al. The number of lung transplants can be safely doubled using extended criteria donors; a single-center review. Transpl Int 2010;23:628-35. [PubMed]
- Pierre AF, Sekine Y, Hutcheon MA, et al. Marginal donor lungs: a reassessment. J Thorac Cardiovasc Surg 2002;123:421-7; discussion, 427-8. [PubMed]
- Gabbay E, Williams TJ, Griffiths AP, et al. Maximizing the utilization of donor organs offered for lung transplantation. Am J Respir Crit Care Med 1999;160:265-71. [PubMed]
- Lardinois D, Banysch M, Korom S, et al. Extended donor lungs: eleven years experience in a consecutive series. Eur J Cardiothorac Surg 2005;27:762-7. [PubMed]
- Straznicka M, Follette DM, Eisner MD, et al. Aggressive management of lung donors classified as unacceptable: excellent recipient survival one year after transplantation. J Thorac Cardiovasc Surg 2002;124:250-8. [PubMed]
- Wilkes DS, Egan TM, Reynolds HY. Lung transplantation: opportunities for research and clinical advancement. Am J Respir Crit Care Med 2005;172:944-55. [PubMed]
- Christie JD, Kotloff RM, Ahya VN, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med 2005;171:1312-6. [PubMed]
- de Perrot M, Liu M, Waddell TK, et al. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med 2003;167:490-511. [PubMed]
- Baldwin MR, Peterson ER, Easthausen I, et al. Donor age and early graft failure after lung transplantation: a cohort study. Am J Transplant 2013;13:2685-95. [PubMed]
- Bittle GJ, Sanchez PG, Kon ZN, et al. The use of lung donors older than 55 years: a review of the United Network of Organ Sharing database. J Heart Lung Transplant 2013;32:760-8. [PubMed]
- Fischer S, Gohrbandt B, Struckmeier P, et al. Lung transplantation with lungs from donors fifty years of age and older. J Thorac Cardiovasc Surg 2005;129:919-25. [PubMed]
- Fessart D, Dromer C, Thumerel M, et al. Influence of gender donor-recipient combinations on survival after human lung transplantation. Transplant Proc 2011;43:3899-902. [PubMed]
- Roberts DH, Wain JC, Chang Y, et al. Donor-recipient gender mismatch in lung transplantation: impact on obliterative bronchiolitis and survival. J Heart Lung Transplant 2004;23:1252-9. [PubMed]
- International Society of Heart and Lung Transplantation Registry, Sato M, Gutierrez C, et al. The effect of gender combinations on outcome in human lung transplantation: the International Society of Heart and Lung Transplantation Registry experience. J Heart Lung Transplant 2006;25:634-7. [PubMed]
- Thabut G, Mal H, Cerrina J, et al. Influence of donor characteristics on outcome after lung transplantation: a multicenter study. J Heart Lung Transplant 2005;24:1347-53. [PubMed]
- Allen JG, Weiss ES, Merlo CA, et al. Impact of donor-recipient race matching on survival after lung transplantation: analysis of over 11,000 patients. J Heart Lung Transplant 2009;28:1063-71. [PubMed]
- Bonser RS, Taylor R, Collett D, et al. Effect of donor smoking on survival after lung transplantation: a cohort study of a prospective registry. Lancet 2012;380:747-55. [PubMed]
- Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 2013;187:527-34. [PubMed]
- Taghavi S, Jayarajan S, Komaroff E, et al. Double-lung transplantation can be safely performed using donors with heavy smoking history. Ann Thorac Surg 2013;95:1912-7; discussion 1917-8.
- Berman M, Goldsmith K, Jenkins D, et al. Comparison of outcomes from smoking and nonsmoking donors: thirteen-year experience. Ann Thorac Surg 2010;90:1786-92. [PubMed]
- Oto T, Griffiths AP, Levvey B, et al. A donor history of smoking affects early but not late outcome in lung transplantation. Transplantation 2004;78:599-606. [PubMed]
- Weill D, Dey GC, Hicks RA, et al. A positive donor gram stain does not predict outcome following lung transplantation. J Heart Lung Transplant 2002;21:555-8. [PubMed]
- Mattner F, Kola A, Fischer S, et al. Impact of bacterial and fungal donor organ contamination in lung, heart-lung, heart and liver transplantation. Infection 2008;36:207-12. [PubMed]
- Campos S, Caramori M, Teixeira R, et al. Bacterial and fungal pneumonias after lung transplantation. Transplant Proc 2008;40:822-4. [PubMed]
- Bonde PN, Patel ND, Borja MC, et al. Impact of donor lung organisms on post-lung transplant pneumonia. J Heart Lung Transplant 2006;25:99-105. [PubMed]
- Bolton JS, Padia SA, Borja MC, et al. The predictive value and inter-observer variability of donor chest radiograph interpretation in lung transplantation. Eur J Cardiothorac Surg 2003;23:484-7. [PubMed]
- McCowin MJ, Hall TS, Babcock WD, et al. Changes in radiographic abnormalities in organ donors: associations with lung transplantation. J Heart Lung Transplant 2005;24:323-30. [PubMed]
- Barnard JB, Davies O, Curry P, et al. Size matching in lung transplantation: an evidence-based review. J Heart Lung Transplant 2013;32:849-60. [PubMed]
- Eberlein M, Reed RM, Bolukbas S, et al. Lung size mismatch and survival after single and bilateral lung transplantation. Ann Thorac Surg 2013;96:457-63. [PubMed]
- Hennessy SA, Hranjec T, Emaminia A, et al. Geographic distance between donor and recipient does not influence outcomes after lung transplantation. Ann Thorac Surg 2011;92:1847-53. [PubMed]
- Fiser SM, Kron IL, Long SM, et al. Influence of graft ischemic time on outcomes following lung transplantation. J Heart Lung Transplant 2001;20:1291-6. [PubMed]
- Gammie JS, Stukus DR, Pham SM, et al. Effect of ischemic time on survival in clinical lung transplantation. Ann Thorac Surg 1999;68:2015-9; discussion 2019-20.
- Kshettry VR, Kroshus TJ, Burdine J, et al. Does donor organ ischemia over four hours affect long-term survival after lung transplantation? J Heart Lung Transplant 1996;15:169-74. [PubMed]
- Winton TL, Miller JD, deHoyos A, et al. Graft function, airway healing, rejection, and survival in pulmonary transplantation are not affected by graft ischemia in excess of 5 hours. Transplant Proc 1993;25:1649-50. [PubMed]
- Glanville AR, Marshman D, Keogh A, et al. Outcome in paired recipients of single lung transplants from the same donor. J Heart Lung Transplant 1995;14:878-82. [PubMed]
- Meyer DM, Bennett LE, Novick RJ, et al. Effect of donor age and ischemic time on intermediate survival and morbidity after lung transplantation. Chest 2000;118:1255-62. [PubMed]
- Bellingham JM, Santhanakrishnan C, Neidlinger N, et al. Donation after cardiac death: a 29-year experience. Surgery 2011;150:692-702. [PubMed]
- De Oliveira NC, Osaki S, Maloney JD, et al. Lung transplantation with donation after cardiac death donors: long-term follow-up in a single center. J Thorac Cardiovasc Surg 2010;139:1306-15. [PubMed]
- De Vleeschauwer S, Van Raemdonck D, Vanaudenaerde B, et al. Early outcome after lung transplantation from non-heart-beating donors is comparable to heart-beating donors. J Heart Lung Transplant 2009;28:380-7. [PubMed]
- Puri V, Scavuzzo M, Guthrie T, et al. Lung transplantation and donation after cardiac death: a single center experience. Ann Thorac Surg 2009;88:1609-14; discussion 1614-5. [PubMed]
- Gomez-de-Antonio D, Campo-Cañaveral JL, Crowley S, et al. Clinical lung transplantation from uncontrolled non-heart-beating donors revisited. J Heart Lung Transplant 2012;31:349-53. [PubMed]
- Snell GI, Levvey BJ, Oto T, et al. Early lung transplantation success utilizing controlled donation after cardiac death donors. Am J Transplant 2008;8:1282-9. [PubMed]
- Arnaoutakis GJ, Sodha NR, Tedford RJ, et al. Centers for Disease Control ‘High-Risk’ Donors and Thoracic Organ Transplantation: Expanding the Donor Pool. J Heart Lung Transplant 2013;32:S123.
- Follette DM, Rudich SM, Babcock WD, et al. Improved oxygenation and increased lung donor recovery with high-dose steroid administration after brain death. J Heart Lung Transplant 1998;17:423-9. [PubMed]
- Zafar F, Khan MS, Heinle JS, et al. Does donor arterial partial pressure of oxygen affect outcomes after lung transplantation? A review of more than 12,000 lung transplants. J Thorac Cardiovasc Surg 2012;143:919-25. [PubMed]
- Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431-40. [PubMed]
- Steen S, Sjöberg T, Pierre L, et al. Transplantation of lungs from a non-heart-beating donor. Lancet 2001;357:825-9. [PubMed]
- Ingemansson R, Eyjolfsson A, Mared L, et al. Clinical transplantation of initially rejected donor lungs after reconditioning ex vivo. Ann Thorac Surg 2009;87:255-60. [PubMed]
- Pêgo-Fernandes PM, de Medeiros IL, Mariani AW, et al. Ex vivo lung perfusion: early report of Brazilian experience. Transplant Proc 2010;42:440-3. [PubMed]
- Wallinder A, Ricksten SE, Hansson C, et al. Transplantation of initially rejected donor lungs after ex vivo lung perfusion. J Thorac Cardiovasc Surg 2012;144:1222-8. [PubMed]
- Zych B, Popov AF, Stavri G, et al. Early outcomes of bilateral sequential single lung transplantation after ex-vivo lung evaluation and reconditioning. J Heart Lung Transplant 2012;31:274-81. [PubMed]
- Aigner C, Slama A, Hötzenecker K, et al. Clinical ex vivo lung perfusion--pushing the limits. Am J Transplant 2012;12:1839-47. [PubMed]
- Whiting D, Banerji A, Ross D, et al. Liberalization of donor criteria in lung transplantation. Am Surg 2003;69:909-12. [PubMed]
- Moreno P, Alvarez A, Santos F, et al. Extended recipients but not extended donors are associated with poor outcomes following lung transplantation. Eur J Cardiothorac Surg 2014;45:1040-7. [PubMed]