Extended-sleeve lobectomy: a technically demanding last-ditch effort in lung sparing surgery for central tumor
Modern surgical treatment of centrally located non-small-cell lung cancer (NSCLC) is based on the principle of parenchyma preservation by the use of technical artifice to avoid pneumonectomy.
The superiority of simple-sleeve lobectomy (SSL) over pneumonectomy was established by Deslauriers et al., in 2004 (1). SSL, the resection of one lobe with standard bronchoplasty produces better outcomes compared to pneumonectomy (2). This technique allows for lower rates of post-operative morbidity and mortality (1), better quality of life after surgery (3), and better global survival (4), higher rate of completed adjuvant treatment (5). SSL is feasible also as a primary treatment after induction chemotherapy (5,6), without increase of morbid-mortality.
Double-sleeve resection (7), pulmonary artery replacement (8) and extended-sleeve lobectomy (ESL) (9) are last-ditch efforts for parenchyma preservation, in the treatment of centrally located tumors not amenable to SSL.
ESL is defined by atypical bronchoplasty with resection of more than one lobe. Four previous publications (9-12) (Table 1) have established the feasibility of ESL.
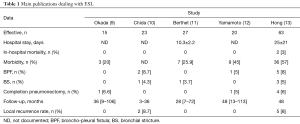
Full table
Those techniques have been performed so far in only a few expert centers as special technical challenges accompany them. (I) Discrepancy between bronchial caliber and shape may be managed through various techniques: caliber reduction of the proximal bronchus (9), telescopic Hollaus anastomosis (14), or oblique resection of the distal bronchus (13) (Figure 1); (II) bronchial stumps vascularization can be preserved through the conservation of a short proximal bronchial stump and the limitation of the dissection around the distal bronchial stump, without coagulation devices, and the conservation of peribronchial tissues; (III) the management of the pulmonary artery is quite demanding during these procedures and could call for double sleeve resection or segmental replacement of the artery, and avoidance of any kinking of the remaining arteries; (IV) the differences between lung volume after resection and chest cavity can be limited by the U-shaped opening of the pericardium around the vein confluence, regular endoscopic cleaning of airways, proper management of chest tubes and non-consensual phrenic palsy to reduce temporarily the thoracic cavity (10).
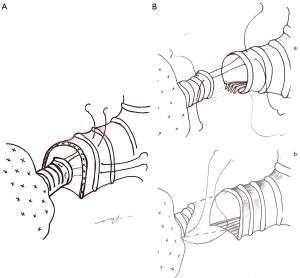
The previous publications cited above (9-12) agree on the reliability of ESL and the absence of surmortality of ESL compared to SSL, requiring however a more aggressive and specialized monitoring of patients. The technical challenge limits the generalization of ESL and the effective of previous studies probably precluding the definitive conclusion of the superiority of ESL over pneumonectomy.
Hong et al. (13) gave a retrospective review of their 20-year institutional experience with ESL. This study, with 63 patients is the widest published to this day. The team is one of the first to use this technique since its first description (15). The aim of Hong et al.’s study (13) is to compare the issues of ESL to these of SSL, in terms of post-operative and survival outcomes. In this series, ESL represents 15% of the bronchoplastic lobectomies performed during the same period (n=540).
Extensive pre-operative work-up was performed with a systematic invasive assessment of suspected metastatic mediastinal lymph nodes. Neo-adjuvant chemoradiotherapy was delivered in the case of cN2 disease in order to have the best chance of performing R0 surgery.
Despite this rigorous pre-operative work-up, the decision to perform ESL was taken intra-operatively for half of the patients.
Associated angioplasty in 21% of ESL cases and 15% of SSL cases (P=0.28) was performed but not arterial replacement. Okada type A ESL was performed in 14 cases, type B in 4, type C in 8 and anastomosis between the right main bronchus in the upper bronchi in 37 patients. Bronchial anastomosis was performed directly after tangential resection of the distal stump, with separate stitches knotted inside the bronchus for the posterior part and outer part of the bronchus for the anterior part of anastomosis. The technique of oblique bronchotomy was chosen to deal with bronchial diameter discrepancy, and the anastomosis wasn’t protected with any flap. Systematic opening of the pericardium around the vein origin was not performed. These specific technical choices are quite original compared to those reported in the four articles published by other teams (9-12) that may partly explain the rate of bronchial and vascular complications.
Anastomotic complications such as bronchopleural fistula (n=5), bronchial stricture (n=3) and pulmonary vein thrombosis (n=2) reach 16% vs. 9% with SSL (P=0.007). They describe 8% of broncho-pleural fistula, with 4 completion pneumonectomies (6%) (Table 1).
Hong et al. (13) achieved R0 resection in 92% of cases, R1 in 6% and R2 in 2%.
After a mean follow-up of 48 months, 8 patients had a loco-regional recurrence, and among them one had a bronchial stump recurrence. Eighteen percent had a distant metastasis. There is no statistical difference between SSL and ESL in term of recurrence in this series.
One of the weaknesses of this study (13) is that they do not compare ESL and pneumonectomy directly, but use the indirect comparison with SSL, considered superior to pneumonectomy.
Despite these limitations, this study does give credit to ESL as reliable alternative to pneumonectomy. It can achieve comparable morbidity, mortality and survival rates as SSL in the treatment of centrally located NSCLC.
ESL can reasonably be proposed for the treatment of centrally located NSCLC as an alternative to pneumonectomy. Some technical points have to be respected: the proximal stump has to be as short as possible, with little distal dissection. Per-operative bronchoscopy has to be performed to be sure of bronchus sealing. Techniques for the management of bronchus discrepancy, space discrepancy and artery kinking are numerous, and each team has to choose the one they master. In our opinion, the systematic use of an intercostal muscular flap has a key role particularly to prevent conflict between bronchoplasty and angioplasty. The level of bronchial resection has to be confirmed by per-operative frozen secretion analysis of the margin, and pneumonectomy is still an option in cases where the surgeon cannot obtain the R0 margin with parenchyma preserving techniques.
The post-operative period present particular risks and justifies regular bronchoscopy as described by Ludwig et al. (16).
Future studies have to answer the question of better prediction of the extension of the resection on pre-operative work-up to lower the rate of unpredicted ESL and pneumonectomy. If this technique of parenchyma preservation could be predicted with more reliability, R0 surgery, with ESL techniques could be proposed for those patients with marginal lung function, who are unable to receive a pneumonectomy.
Acknowledgements
The authors wish to thank Daniel Pop, MD for proofreading.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Deslauriers J, Grégoire J, Jacques LF, et al. Sleeve lobectomy versus pneumonectomy for lung cancer: a comparative analysis of survival and sites or recurrences. Ann Thorac Surg 2004;77:1152-6. [Crossref] [PubMed]
- Shi W, Zhang W, Sun H, et al. Sleeve lobectomy versus pneumonectomy for non-small cell lung cancer: a meta-analysis. World J Surg Oncol 2012;10:265. [Crossref] [PubMed]
- Ferguson MK, Lehman AG. Sleeve lobectomy or pneumonectomy: optimal management strategy using decision analysis techniques. Ann Thorac Surg 2003;76:1782-8. [Crossref] [PubMed]
- Park JS, Yang HC, Kim HK, et al. Sleeve lobectomy as an alternative procedure to pneumonectomy for non-small cell lung cancer. J Thorac Oncol 2010;5:517-20. [Crossref] [PubMed]
- Gómez-Caro A, Boada M, Reguart N, et al. Sleeve lobectomy after induction chemoradiotherapy. Eur J Cardiothorac Surg 2012;41:1052-8. [Crossref] [PubMed]
- Toyooka S, Soh J, Yamamoto H, et al. Extended sleeve lobectomy after induction chemoradiotherpy for non-small cell lung cancer. Surg Today 2015;45:1121-6. [Crossref] [PubMed]
- Rendina EA, De Giacomo T, Venuta F, et al. Lung conservation techniques: bronchial sleeve resection and reconstruction of the pulmonary artery. Semin Surg Oncol 2000;18:165-72. [Crossref] [PubMed]
- Berthet JP, Boada M, Paradela M, et al. Pulmonary sleeve resection in locally advanced lung cancer using cryopreserved allograft for pulmonary artery replacement. J Thorac Cardiovasc Surg 2013;146:1191-7. [Crossref] [PubMed]
- Okada M, Tsubota N, Yoshimura M, et al. Extended sleeve lobectomy for lung cancer: the avoidance of pneumonectomy. J Thorac Cardiovasc Surg 1999;118:710-3. [Crossref] [PubMed]
- Chida M, Minowa M, Miyoshi S, et al. Extended sleeve lobectomy for locally advanced lung cancer. Ann Thorac Surg 2009;87:900-5. [Crossref] [PubMed]
- Berthet JP, Paradela M, Jimenez MJ, et al. Extended sleeve lobectomy: one more step toward avoiding pneumonectomy in centrally located lung cancer. Ann Thorac Surg 2013;96:1988-97. [Crossref] [PubMed]
- Yamamoto K, Miyamoto Y, Ohsumi A, et al. Sleeve lung resection for lung cancer: analysis according to the type of procedure. J Thorac Cardiovasc Surg 2008;136:1349-56. [Crossref] [PubMed]
- Hong TH, Cho JH, Shin S, et al. Extended sleeve lobectomy for centrally located non-small-cell lung cancer: a 20-year single-centre experience. Eur J Cardiothorac Surg 2018;54:142-8. [Crossref] [PubMed]
- Hollaus PH, Janakiev D, Pridun NS. Telescope anastomosis in bronchial sleeve resections with high-caliber mismatch. Ann Thorac Surg 2001;72:357-61. [Crossref] [PubMed]
- Johnston JB, Jones PH. The treatment of bronchial carcinoma by lobectomy and sleeve resection of the main bronchus. Thorax 1959;14:48-54. [Crossref] [PubMed]
- Ludwig C, Stoelben E. A new classification of bronchial anastomosis after sleeve lobectomy. J Thorac Cardiovasc Surg 2012;144:808-12. [Crossref] [PubMed]


