Extended sleeve lobectomy: its place in surgical therapy for centrally located non-small cell lung cancer and a review of technical aspects
Sleeve lobectomy has been a well-established and widely accepted alternative to pneumonectomy for centrally located non-small cell lung cancers (NSCLCs) as it is more sparing of lung parenchyma. Until the early 2000s much literature, including a meta-analysis study, described favorable results for sleeve lobectomy, suggesting a comparable oncological outcome to pneumonectomy with lower postoperative mortality and morbidity. Sleeve lobectomy was also associated with better quality of life, by virtue of preserving lung parenchyma (1-9). Consequently, recent guidelines from the American College of Chest Physicians recommend sleeve lobectomy rather than pneumonectomy in patients with clinical early-stage central NSCLC in whom complete resection can be achieved (10).
However, at the time, no prospective randomized trials comparing sleeve lobectomy and pneumonectomy had been performed. Therefore, the ideal surgical procedure for centrally located NSCLC remained controversial, and the indications depended on each case and particularly on the operating surgeon. More recently, in an attempt to answer the question, several nationwide studies with large patient numbers that have compared sleeve lobectomy and pneumonectomy have been published (11,12). Pagès et al. evaluated outcomes after sleeve lobectomy and pneumonectomy using data from the French nationwide database EPITHOR (11). In the study, 6,259 patients underwent sleeve lobectomy or pneumonectomy for NSCLC in 103 centers in France between 2005 and 2014. Statistically, 2 propensity score (PS) techniques, PS matching, and inverse probability for treatment weighting (IPTW) analysis, were indicated to neutralize potential confounding variables. These well-balanced analyses with large cohorts concluded that sleeve lobectomy was not associated with any significant difference in postoperative mortality [4.99% in the sleeve lobectomy group, 5.89% in the pneumonectomy group; P=0.279; odds ratio (of postoperative mortality) associated with pneumonectomy, 1.24 for matching, and 0.77 for IPTW]. However, association was recorded with a significant increase in the rate of pulmonary complications (e.g., atelectasis or pneumonia), and a significant decrease in the rate of bronchopleural fistula (BPF) and empyema compared with pneumonectomy. In terms of survival analysis, only the PS matching technique (but not the IPTW analysis) found that sleeve lobectomy was associated with improved 3-year overall survival and disease-free survival when compared with pneumonectomy. They concluded that, when technically possible, surgeons must perform sleeve lobectomy to provide improved long-term survival benefits to patients even when there may be a risk of more postoperative pulmonary complications.
Another nationwide study comparing outcomes between sleeve lung resections and pneumonectomy was reported from the United States (12). A total of 23,964 patients, of whom 1,713 (7.1%) underwent a sleeve resection and 22,251 (92.9%) underwent a pneumonectomy at 644 hospitals from 1998 to 2012, were included. Similar to the French study, short-term and long-term outcomes were compared using PS matching to minimize potential selection bias and confounding factors between groups. Results showed that sleeve resections were associated with a lower postoperative mortality compared to pneumonectomy, 1.6% vs. 5.9% (P<0.001) at 30 days and 4% vs. 9.4% (P<0.001) at 90 days, respectively. Regarding long-term outcome, the overall survival for patients undergoing sleeve resection was statistically better than for those undergoing pneumonectomy (P<0.001). Additionally, they described that this survival advantage was mostly realized in the first 18 months after surgery. This US study also included an analysis based on hospital-level metrics. A sleeve-to-pneumonectomy (S:P) ratio was used as a quality metric for hospitals; however, the hospital S:P ratios were not associated with postoperative outcomes.
Cusumano et al. from Italy reported further evidence on sleeve lobectomy for central NSCLC (13). They concluded that sleeve lobectomy represented a safe and effective surgical procedure compared with pneumonectomy even after induction therapy including chemoradiotherapy, with substantially comparable short-term and long-term results. Thirty-day mortality and morbidity rates were 3.9% and 9.8% for sleeve lobectomy and 2.9% and 22.1% for pneumonectomy, respectively. Five-year survival rates were 53.8% after sleeve lobectomy and 43.1% after pneumonectomy, respectively (P=0.28). Overall recurrence rate was 42.8% after sleeve lobectomy and 47.0% after pneumonectomy (P=0.34); relapse was locoregional in 22.4% of sleeve lobectomy cases and 12.1% after pneumonectomy, respectively (P=0.011).
Given the results mentioned above, we suggest that sleeve lobectomy should be the preferred procedure for centrally located NSCLC when technically possible. Nevertheless, in reality, less than 10% of pneumonectomy patients underwent sleeve resections in the US, and similarly less than 20% in France (11,12). Sleeve lobectomy is generally a more complex and technically demanding procedure than pneumonectomy, requiring more experienced surgeons. Therefore, it is inferred that the number of experienced surgeons and institutions performing sleeve lobectomy is still limited, and pneumonectomy is therefore more commonly chosen for centrally located NSCLC.
Now we come to the main topic of this article, Hong et al. reported their institutional results of extended sleeve lobectomy (ESL) for centrally located NSCLC in 2017 (14). ESL was initially described as a pulmonary resection of more than 1 lobe with atypical bronchial resection and reconstruction by Okada et al. (15). Many years previously in 1959, Johnston et al. had described their experiences and the feasible short-term outcome of sleeve lobectomy including procedures of ESL (16). Compared with standard sleeve lobectomy, which is defined as a resection of one lobe with simple bronchial resection and reconstruction, several technical difficulties could occur in ESL; these include a greater size discrepancy in bronchial calibers, increased anastomotic site tension, and more frequent combined angioplasty. Hong et al. demonstrated both the feasible postoperative results and the long-term outcome of ESL at their institution in comparison with standard sleeve lobectomy (14). So far, several other ESL studies have also demonstrated similar feasible short-term and long-term outcomes; however, the details in each report differ (15,17-20). Results from all of the studies, including Hong’s paper, are summarized in Table 1. Although each study includes a limited number of patients (9–63 patients), Hong’s research contains the largest cohort and is most current. Yamamoto et al. (17) reviewed all their experience of sleeve resection, and only 20 of 201 were defined as ESL using their own definition. Induction therapy was not frequently indicated in Hong’s study (6.3%) compared to all others (17.4–100%). Toyooka et al. focused on patients after induction chemoradiation consisting of platinum doublet chemotherapy with concurrent radiotherapy up to 40–46 Gy.
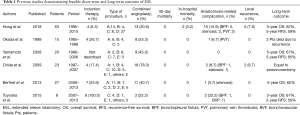
Full table
Regarding the type of procedure, ESL protocol in each article was classified again according to Okada with small modifications. Okada et al. originally classified ESL into the following 3 types; a resection of the right upper and middle lobe ± S6 with a reconstruction between the right main bronchus and the lower lobe or basal segment bronchus was defined as type A; type B was a resection of the left upper lobe and S6 with a reconstruction between the left main bronchus and the basal segment bronchus; and type C was a resection of the left lower lobe and lingular segment with a reconstruction between the left main bronchus and the segmental bronchus of the upper tri-segments (15). Unlike in Okada et al., the other studies included a resection of the right middle and the lower lobe with a reconstruction between the right main bronchus and the upper lobe bronchus as an ESL, and a resection of the right upper lobe and S6 with a reconstruction between the right main bronchus and an orifice of the middle lobe and basal segment was also included (14,17-20). In the current article, the latter are classified as type D and type E of the modified Okada’s classification, respectively. Illustrations of each type of modified Okada’s classification are shown in Figure 1. Characteristics of the ESL procedure in each study are as follows: Hong et al. included a larger portion of type D procedures compared to other studies (14); Yamamoto et al. did not include simple sleeve bi-lobectomy, so most of the type A and type D procedures were not defined as ESL (17); the 20 ESL cases consisted of 2 of type A, 8 of type B, 7 of type C, and 3 of type E; Chida et al. and Toyooka et al. included several cases of carinal plasty or tracheal plasty as other types of procedure (18,20); however, the latter are normally indicated in more central diseases and have different technical aspects; accordingly, they should be considered separately from ESL in the current article.
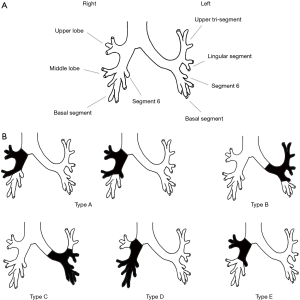
From a surgical point of view, each type of ESL has its own characteristics. Type A procedures normally require a long bronchial resection from the level of the right main bronchus to the level of the basal segment bronchus. As a result, management to reduce tension associated with anastomosis is quite important in the prevention of anastomotic complications. In addition to blunt dissection of the peribronchial tissue around an anastomotic site, hilar release by cutting the pericardium around the inferior pulmonary vein is useful in cases of high tension anastomosis and was performed by most authors (14,15,18-20). From an anatomical perspective, combined angioplasty of the pulmonary artery would frequently be required in type A. The extent of bronchial resection is also large in type B procedures. The features of type B are similar to those of type A, such as high-tension anastomosis and high frequency of combined pulmonary artery reconstruction. Type C has different technical aspects. A large size discrepancy easily occurs between the proximal and distal bronchial stump; therefore, careful caliber adjustment in anastomosis more important than anastomotic tension management and combined angioplasty. As to techniques of caliber adjustment, telescope anastomosis (17,18,20) and the placing of adjustment sutures in membranous parts of the proximal bronchus (15,19) may be useful. Similar to type C procedures, type D require techniques of caliber adjustment. Furthermore, the preservation of lung parenchyma in both type C and type D can be less than in other procedure types. For that reason, management for residual space in the chest cavity might be important in preventing space related problems such as empyema and airway-pleural fistula. Hong et al. and Berthet et al. described how the meticulous management of chest drainage was necessary, and Chida et al. mentioned artificial phrenic nerve palsy to reduce the space (14,18,19). The features of type E are almost the same as type A; however, resection of the intersegmental plane should be performed carefully, not too close to the tumor, to achieve an R0 resection.
Moving on to more technical details, 4-0 absorbable monofilament suture was the commonly used suture material for anastomosis (15,19,20); however, 4-0 or 3-0 absorbable braided suture, and 3-0 absorbable monofilament suture were also used according to the author’s preference (14,17,18). As to suturing method, the interrupted fashion was used in four papers (14,15,17,18), whereas Berthet et al. and Toyooka et al. adopted a hybrid technique of continuous running fashion and interrupted fashion (19,20). In terms of coverage of the anastomotic site, Berthet et al. used the intercostal muscle flap routinely (19), and Toyooka et al. usually used pericardial fat tissue or greater omentum for anastomosis covering after chemoradiation (20). A further 4 authors did not perform anastomosis coverage routinely, but Okada et al., Yamamoto et al., and Chida et al. created coverage with fat tissue or muscle only in cases with combined angioplasty to separate two anastomotic sites, thus preventing bronchovascular fistulae (BVF) (15,17,18). Despite precise surgical efforts, life-threatening complications such as BPF, BVF, and pulmonary vein thrombosis (PVT) were observed as anastomosis-related complications in several series. In Hong’s series, BPF was more frequently observed compared to other series; however, any correlation between complications and surgical techniques is uncertain. PVT may not be a directly anastomosis-related complication; however, it can occur as a result of overstretching of the pulmonary vein. In particular, tension management using pericardial cutting may be useful for preventing this complication after a type A or type B procedure. In several cases with BPF or PVT, completion pneumonectomy was required to control the complication (14,15,20). Appropriate management for postoperative complications are also necessary to achieve both feasible short-term results and long-term outcomes.
As a result of the precise surgical techniques and careful perioperative management for overcoming the difficulties associated with ESL, all studies reported excellent short-term surgical results with no 30-day mortality (0%), and with only two in-hospital deaths (3.2%) observed in Hong’s study (14,15,17-20). Furthermore, the long-term results were also satisfactory with 62–67% of overall survival at 5 years and with 56–65% of recurrence-free survival at 5 years (14,17,19). All authors concluded ESL was a safe and feasible procedure, and an appropriate alternative to pneumonectomy in patients with centrally located tumors.
All studies were retrospective in nature with a limited number of patients, which may have led to substantial selection bias and poor statistical power. Although the direct comparison of outcomes with other studies lacks reliability, these results approximate the most recent outcomes of sleeve lobectomy and are superior to the outcomes of pneumonectomy (11,12). Accordingly, we partly agree with the findings that ESL is a safe and feasible procedure for central NSCLC. However, that is only in highly selected institutions with experienced surgeons and only for appropriate surgical and oncological patients. Despite the results from the studies given in Table 1, ESL potentially carries more risk of surgical and oncological factors and results in a lower volume of preserved lung tissue than standard sleeve lobectomy. “Sleeve lobectomy or Pneumonectomy?” still remains a question of procedure for centrally located NSCLC. Pneumonectomy can be the best surgical option on occasions. Procedures with many potential risks and poor benefits must be avoided. Each surgeon needs to carefully judge the selection of the correct surgical procedure, and commit to developing their skill and techniques to perform ESL safely.
Acknowledgements
Funding: The study was supported by a grant from Sougou-Kagaku Kennkyu team of Fukuoka University (181043).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gaissert HA, Mathisen DJ, Moncure AC, et al. Survival and function after sleeve lobectomy for lung cancer. J Thorac Cardiovasc Surg 1996;111:948-53. [Crossref] [PubMed]
- Okada M, Yamagishi H, Satake S, et al. Survival related to lymph node involvement in lung cancer after sleeve lobectomy compared with pneumonectomy. J Thorac Cardiovasc Surg 2000;119:814-19. [Crossref] [PubMed]
- Deslauriers J, Grégoire J, Jacques LF, et al. Sleeve lobectomy versus pneumonectomy for lung cancer: a comparative analysis of survival and sites or recurrences. Ann Thorac Surg 2004;77:1152-6; discussion 1156. [Crossref] [PubMed]
- Ludwig C, Stoelben E, Olschewski M, et al. Comparison of morbidity, 30-day mortality, and long-term survival after pneumonectomy and sleeve lobectomy for non-small cell lung carcinoma. Ann Thorac Surg 2005;79:968-73. [Crossref] [PubMed]
- Kim YT, Kang CH, Sung SW, et al. Local control of disease related to lymph node involvement in non-small cell lung cancer after sleeve lobectomy compared with pneumonectomy. Ann Thorac Surg 2005;79:1153-61; discussion 1153. [Crossref] [PubMed]
- Bagan P, Berna P, Pereira JC, et al. Sleeve lobectomy versus pneumonectomy: tumor characteristics and comparative analysis of feasibility and results. Ann Thorac Surg 2005;80:2046-50. [Crossref] [PubMed]
- Takeda S, Maeda H, Koma M, et al. Comparison of surgical results after pneumonectomy and sleeve lobectomy for non-small cell lung cancer: trends over time and 20-year institutional experience. Eur J Cardiothorac Surg 2006;29:276-80. [Crossref] [PubMed]
- Park JS, Yang HC, Kim HK, et al. Sleeve lobectomy as an alternative procedure to pneumonectomy for non-small cell lung cancer. J Thorac Oncol 2010;5:517-20. [Crossref] [PubMed]
- Shi W, Zhang W, Sun H, et al. Sleeve lobectomy versus pneumonectomy for non-small cell lung cancer: a meta-analysis. World J Surg Oncol 2012;10:265. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Pagès PB, Mordant P, Renaud S, et al. Sleeve lobectomy may provide better outcomes than pneumonectomy for non-small cell lung cancer. A decade in a nationwide study. J Thorac Cardiovasc Surg 2017;153:184-95.e3. [Crossref] [PubMed]
- Abdelsattar ZM, Shen KR, Yendamuri S, et al. Outcomes after sleeve lung resections versus pneumonectomy in the United States. Ann Thorac Surg 2017;104:1656-64. [Crossref] [PubMed]
- Cusumano G, Marra A, Lococo F, et al. Is sleeve lobectomy comparable in terms of short- and long-term results with pneumonectomy after induction therapy? A multicenter analysis. Ann Thorac Surg 2014;98:975-83. [Crossref] [PubMed]
- Hong TH, Cho JH, Shin S, et al. Extended sleeve lobectomy for centrally located non-small-cell lung cancer: a 20-year single-centre experience. Eur J Cardiothorac Surg 2018;54:142-8. [Crossref] [PubMed]
- Okada M, Tsubota N, Yoshimura M, et al. Extended sleeve lobectomy for lung cancer: the avoidance of pneumonectomy. J Thorac Cardiovasc Surg 1999;118:710-3; discussion 713-4. [Crossref] [PubMed]
- Johnston JB, Jones PH. The treatment of bronchial carcinoma by lobectomy and sleeve resection of the main bronchus. Thorax 1959;14:48. [Crossref] [PubMed]
- Yamamoto K, Miyamoto Y, Ohsumi A, et al. Sleeve lung resection for lung cancer: analysis according to the type of procedure. J Thorac Cardiovasc Surg 2008;136:1349-56. [Crossref] [PubMed]
- Chida M, Minowa M, Miyoshi S, et al. Extended sleeve lobectomy for locally advanced lung cancer. Ann Thorac Surg 2009;87:900-5. [Crossref] [PubMed]
- Berthet JP, Paradela M, Jimenez MJ, et al. Extended sleeve lobectomy: one more step toward avoiding pneumonectomy in centrally located lung cancer. Ann Thorac Surg 2013;96:1988-97. [Crossref] [PubMed]
- Toyooka S, Soh J, Yamamoto H, et al. Extended sleeve lobectomy after induction chemoradiotherapy for non-small cell lung cancer. Surg Today 2015;45:1121-6. [Crossref] [PubMed]


