Adjuvant, neoadjuvant, and definitive radiation therapy for malignant pleural mesothelioma
Introduction
Malignant pleural mesothelioma (MPM) can present with a variety of symptoms, including dyspnea due to an effusion and/or mass effect from pleural tumor, pain and anorexia. The care of MPM patients is by necessity multidisciplinary in nature because no single therapy is sufficient to manage most patients throughout the course of their disease. Although there is significant variation with important prognostic variables such as age, stage at presentation and histologic subtype, median survivals for patients with MPM generally range from 6–14 months.
External beam radiation therapy (RT) is frequently used with palliative intent to relieve symptoms arising from compression or invasion of normal structures/organs. Other palliative therapies include physiologically-directed therapies such as drainage of pleural effusions, medically-directed therapies such as chemotherapy, and symptom-directed therapies such as narcotic analgesics. Anatomically, the pleura and primary MPM tumor bulk are distributed over a large surface area that surrounds the relatively radiosensitive lung parenchyma and borders the heart, great vessels, esophagus, proximal bronchial tree, and spinal cord. While the local response rates for RT meet or far exceed those of chemotherapy, the intrinsic radiosensitivity of these normal organs limits the area that can be safely treated with RT in the palliative setting. Nevertheless, technological advances in RT delivery have extended its use in symptom management for patients with MPM. Definitive management of multidisciplinary MPM typically involves surgical cytoreduction and can be used to increase local control and allow lung-sparing procedures to be performed.
Fundamentals of RT
The therapeutic index of RT represents a composite of both spatial/anatomical dose distribution and the radiobiological properties of the affected cells/tissues. Spatial selectivity for cancer tissues over normal tissues is achieved by using an appropriate form of RT (electrons, photons, protons) and modulating the fluence/energy profile of ionizing radiation delivered to create dose distributions in which the tumor tissues receive more dose than the surrounding normal tissues. Cancer selectivity on a cellular level is achieved by fractionating the radiation dose over days to weeks, typically delivering one radiation dose per weekday, and increasing the number of fractions to mitigate the risk of radiation-induced normal tissue toxicity for increasing volumes of irradiated normal tissues and/or highly radiosensitive adjacent normal tissues. For the largest tumor volume in which the entire external ipsilateral pleura is treated, an increasingly commonly used RT regimen is 28 fractions to deliver a 50.4-Gy dose to a large proportion of the pleura. This has a 15–20% risk of grade 1–3 complications (1). For highly conformal treatment of relatively small areas, stereotactic body radiotherapy (SBRT) techniques can be used to deliver to a total dose of 50–60 Gy with photons in 3–5 fractions with a low (5–10%) risk of grade 1–3 complications and a very low (~1%) risk of grade 4–5 complications (2).
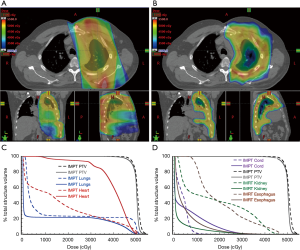
In terms of technique, more basic forms of photon RT are delivered using either 2D or 3D conformal treatment planning that consist of geometric field shapes (portals) defined by multileaf collimator (MLC) blocks delivered from 1–4 beam angles. More complex photon dose distributions are achieved by modulating the fluence across each portal using multiple different MLC configurations per portal (intensity-modulated radiation therapy, IMRT) to create highly conformal dose distributions with convex or concave geometry, and additional conformality can be achieved by moving the gantry in a rotational arc whereas the MLC can be adjusted continuously while the beam is delivered in a technique called volumetric modulated arc RT (VMAT). In both cases, the increased conformality of these dose gradients is achieved at the cost of increased volume of normal tissues receiving a lower RT dose. Other mechanical solutions for modulating photon RT doses include CyberKnife and TomoTherapy, but these also suffer from the fundamental problem of increased low dose to normal tissues derived from the physical characteristics of photons. The major spatial issue for patients with MPM is that the relative radiosensitivity of the normal thoracic organs does not permit the delivery of an effectively tumoricidal RT dose to all pleural surfaces using conventional RT (photons and electrons) while preserving normal/sufficient cardiopulmonary function. Because protons have different physical characteristics in tissues (i.e., the potential for no exit dose), proton RT has the potential to provide a greater degree of spatial control over photon dose distribution and permits the delivery of increasing doses of ionizing radiation to malignant tumors while sparing dose to critical normal tissues for thoracic tumors (Figure 1). Early reports of proton RT as a part of a multimodality management schema (including sequential chemotherapy) demonstrates the potential to achieve dramatic and long lasting clinical benefits for patients with MPM (3,4). Attempting to treat the entirety of the pleura, including the major/minor fissures, typically results in very little dose sparing for the ipsilateral lung. Thus, in clinical trials of pleural RT with intact lung, RT has been typically limited to treatment of the peripheral pleural in the definitive or adjuvant settings or to treating specific, symptomatic regions in the palliative setting.
RT and surgical cytoreduction
To address both the widespread local disease and the substantial risk of systemic disease, multiple standard treatment modalities are typically combined in definitive approaches to managing patients with MPM. Nevertheless, the ability to combine highly aggressive local and systemic treatment strategies can be limited by the relative potential morbidities of these strategies. In patients with good performance status and few comorbidities, RT with definitive intention (and higher RT doses) can lead to significantly higher median survivals than have been reported with palliative therapies. Because no single modality by itself is wholly effective in the definitive management of MPM, the strategy in any surgery-based multimodal treatment plan is to use surgery to achieve a macroscopic complete resection and to then employ other modalities such as intraoperative adjuvants in an attempt to control the inevitably present residual microscopic disease. In the absence of surgical resection, RT has been used definitively in selected patients to treat bulky areas of disease or even all glycolytically active (FDG-avid) disease (5). However, even with protons, it is often difficult to deliver high dose RT to all pleural surfaces without unacceptable toxicities to the intact lung underneath. Thus, definitive RT can instead be performed in an adjuvant fashion after surgical cytoreduction performed with the goal of achieving a macroscopically complete resection (MCR) that reduces the total dose required to achieve durable local control. One strategy for MCR involves an extrapleural pneumonectomy (EPP), in which the parietal pleura, diaphragm, pericardium and lung are resected en bloc. The other commonly used strategy is the lung-sparing pleurectomy/decortication (P/D), which when performed with the intent of achieving an MCR is often referred to as an extended P/D (eP/D) or radical pleurectomy. There is currently no procedure acknowledged as the “standard of care” cytoreductive operation for patients with pleural malignancies, and both EPP and eP/D are performed at high volume MPM surgical centers (6,7). Following surgical cytoreduction by either EPP or eP/D, hemithoracic RT has been used in an attempt to diminish the risk of local recurrences.
Adjuvant hemithoracic RT following EPP
A number of techniques for adjuvant irradiation of the ipsilateral chest wall following EPP have been described. The entire hemithorax can be treated to a total dose of 30–40 Gy using 3D conformal photon RT techniques with a photon boost used to treat high-risk regions to total doses of 50–60 Gy. Data on 3D techniques demonstrate significant acute, subacute and chronic toxicities, including radiation pneumonitis, pulmonary fibrosis, pulmonary vascular damage, esophagitis, pericarditis, and pneumothorax despite generally sub-therapeutic doses to much of the treatment volume. More modern techniques include mixed photon-electron (8) and hemithoracic IMRT techniques (9). As is typically the case with conventional RT treatments, increased conformality comes at the cost of increased exposure of normal tissues to a low dose of RT (“low dose wash” effect). With the mixed photon-electron technique, there is a decrease in the volume of contralateral lung exposed to lower RT doses (10), while the use of IMRT or VMAT approaches have increased conformality and consistency in delivery but increased dose to the contralateral lung and heart. Despite the potential for simplicity and reproducibility with the inverse treatment planning process, there remains a significant learning curve for treatment planning using these techniques, with experienced centers demonstrating clearly superior plans in terms of dose to target vs. normal tissues (11).
Studies of definitive RT to the involved hemithorax following EPP overall demonstrate improved local control when compared to studies of surgery alone, with serious but potentially tolerable toxicities. The typical protocol consists of neoadjuvant chemotherapy, EPP and post-operative hemithoracic RT, and results have been replicated across a number of centers in Europe, Asia and North America (12-19) (Table 1). In these studies, about 2/3 of the patients were earlier stage (AJCC 7th edition stage I/II) MPM patients generally treated with total doses of 50–60 Gy in 1.8–2 Gy fractions. When analyzed by an intent to treat that includes all patients who started neoadjuvant chemotherapy in the survival analysis, this approach yields a median overall survival ranging from 14–20 months. When analyzed by methods that exclude patients who did not complete the full course of trimodality treatment, median overall survivals are significantly improved to 15–40 months. However, 20–25% of patients did not undergo surgery primarily due to disease progression on chemotherapy (EPP column in Table 1), and only 40–60% of patients completed the full trimodality course (RT column in Table 1). Note that in one trial, 46% of patients developed radiation pneumonitis, and analysis of these data suggests that low-dose spread to the contralateral lung can result in potentially fatal pulmonary toxicity (20). This clearly demonstrates the relatively steep learning curve required to produce RT plans that minimize the dose to normal tissues, but even patients treated at experienced centers have significant risks of radiation pneumonitis that is fatal in 3–10% of cases.
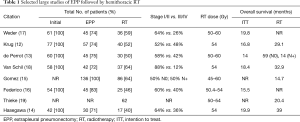
Full table
As noted above, these data have been compared in a non-randomized, retrospective fashion to institutional and published data to suggest that patients who complete RT have superior progression free and overall survival when compared to patients who only complete EPP. Since patients who undergo EPP without RT are often too moribund to receive RT or have rapidly progressive disease, there is an intrinsic potential for bias in these comparisons. To quantify the potential magnitude of benefit of post-EPP RT, the Swiss Group for Clinical Cancer Research (SAKK) conducted a randomized clinical trial of preoperative chemotherapy followed by EPP with or without RT at 14 centers in Switzerland, Belgium and Germany (21). In this trial, 151 patients with stage I–III MPM received preoperative chemotherapy consisting of cisplatin/pemetrexed every 21 days for 3 cycles, with 113 patients (75%) continuing to EPP. Of the patients who underwent EPP, an 85% MCR rate (96/113) was achieved that represented 64% of the original 151 patients who started chemotherapy. Fifty-four of these 96 patients (56%) underwent randomization to observation vs. postoperative IMRT (27 in each group or 18% of the original patients). Final analysis of these data failed to demonstrate a statistically significant improvement in overall survival. Critiques of this study noted that the RT cohort consisted of only 27 patients out of the original 151 who were treated at 14 centers with 3 different dose/fractionation schemes without central RT quality assurance and also noted the aforementioned impact of center RT experience on quality of plans (22). Moreover, the OS for all 151 patients was on the lower end of the intention to treat overall survivals noted in Table 1 at 15 months. Nevertheless, these findings, along with the generally superior results of protocols using lung-sparing surgery, raise a cautionary note for the future of EPP/RT in the management of patients with MPM.
Adjuvant hemithoracic RT following eP/D
With these results and the trend in surgical management toward lung sparing surgical cytoreduction, there is an increasingly urgent need to develop additional adjuvant technologies to reduce the rate of local recurrence. PDT, hyperthermic chemotherapy, and hyperthermic povidone iodine can be used intraoperatively (23-25). As noted above, postoperative RT cannot easily be delivered to all pleural surfaces, including the major/minor fissures, even with the most advanced RT techniques/technology. However, investigators at Memorial Sloan Kettering Cancer Center have pioneered a technique using IMRT to treat the peripheral pleural space that carries the highest risk of local recurrence detailed in the recently published IMPRINT study. In this study, 45 patients with MPM were treated with neoadjuvant chemotherapy with 21 patients (47%) proceeding to lung-sparing surgery and 16 (36%) proceeding to post-operative RT (1). An additional 11 patients (24%) went on to RT without surgical resection for a total of 27 patients (60%) who received RT to the external pleural surfaces as depicted in Figure 1A. Of the patients who received RT, the median dose was 46.8 Gy and the median overall survival was 23.7 months. Comparable results have been reported by other groups, with 20 patients experiencing a 33-month median overall survival in one trial and 20 patients experiencing a median overall survival of 28.4 months in another (26,27). Moreover, the toxicity rates for IMRT to the external pleura are generally lower than what has been reported previously with older RT delivery techniques. Taken together with the median survivals that are comparable or perhaps superior to those achieved with EPP and RT, these results suggest that RT to the external pleura has potential clinical application both in the operative/adjuvant and non-operative settings.
Neoadjuvant hemithoracic RT followed by EPP
Preoperative RT is standardly performed with significant success in multiple thoracic and extra-thoracic primary tumors, including colorectal, esophageal and lung cancers. Due to concerns over cardiopulmonary toxicity and increased operative mortality, this approach had not been applied to patients with MPM. Using a trial design that is similar to previous trials of hypofractionated, preoperative RT for patients with rectal cancer, de Perrot and colleagues initiated a trial in which patients received 25 Gy in 5 daily fractions to the entire hemithorax with a simultaneous infield boost of 5 Gy to areas of high risk disease (28) to test the feasibility and outcomes of this approach in patients with clinically node negative MPM. Due to concerns over cardiopulmonary toxicity, RT was followed almost immediately (6 days) later by EPP. In the intention to treat analysis for all subjects (epithelial and non-epithelial MPM), the median overall survival was 36 months. In patients with epithelial MPM, the median overall survival was 51 months. Although this number should be interpreted with some caution since the majority of patients are censored prior to 3 years, these results suggest that the fully mature data will continue to demonstrate the potential clinical promise of this approach for clinically node negative patients.
Adjuvant prophylactic RT to interventional sites
Clinically, MPM has a propensity to recur in the chest wall and subcutaneous tissues after interventional procedures such as biopsy, thoracoscopy or thoracotomy. Superficial (orthovoltage X-rays or electrons) RT is often employed in prophylactic irradiation of interventional tracts (PIT) in an attempt to reduce this risk. In the absence of RT, the reported rates of intervention site metastases demonstrate significant variability, with the control (no prophylactic radiation) arm of the three published, randomized clinical trials of PIT showing 10–40% of patients developing intervention site metastases (29-31) (Table 2). However, the ability of PIT to reduce the rate of intervention site recurrence remains an open question, with these randomized trial data demonstrating conflicting results. While many radiation oncologists continue to recommend PIT, more study is needed to identify which patients might benefit most from this therapy. A multicenter randomized phase 3 trial of PIT vs. observation was initiated, and when reported (32), given its size, this trial should significantly improve our understanding of the potential benefits of PIT in patients with MPM.

Full table
Symptom management and palliative RT in patients with MPM
Patients with MPM can experience severe pain, the etiology of which is likely to be multifactorial. After a careful review of the patient’s pain symptoms and the available cross-sectional imaging, areas of invasion into structures such as the chest wall, spinal nerve roots/intercostal nerves or diaphragm by tumor can be identified as anatomic correlates of regional/localized pain. Pain can also arise from malignant pleural effusion, diffuse pleural involvement by MPM or contracture of the pleural tumor rind leading to impingement related pain as the ipsilateral ribs are drawn closer together. Patients also frequently experience dyspnea that is similarly multifactorial, arising from factors such as compressive atelectasis of the lung by a tumor mass and/or pleural effusion, decreased lung compliance/ventilation with a restrictive pattern due to circumferential involvement of lung by tumor, and alteration in ventilation perfusion matching. In general, palliative RT aims to identify and alleviate the most proximal or significant cause of distress and can be highly successful, especially for symptoms with good anatomic correlates on cross sectional imaging caused by a limited extent of the total tumor burden.
Even though RT is a standard of care for palliation of symptoms in patients with MPM, there are few robust studies in the literature that describe response rates or define the optimal RT dose/fractionation. Indeed, despite the known sensitivity of MPM cells to RT, the response rate has rarely been formally reported. One retrospective study of RT in 54 patients with advanced MPM reported an in-field radiologic response rate of 43% at 2 months, which compares favorably with objective response rates for multi-agent chemotherapy (33). Since MPM tumors can take several months to reach minimal size following RT, this 2-month time point may actually underestimate the true objective response rate for RT. It is also important to note that a decrease in painful symptoms is frequently reported by patients prior to, or in the absence of, objective tumor shrinkage. This can be seen in the comparison of the 43% 2-month response rate noted above with a systematic review of the largely retrospective or descriptive studies present in the literature in which the reported RT response rates for pain range from 50–69% (34). To better measure response rates and help define a more standard approach to palliative RT in patients with MPM, Macleod and colleagues undertook a phase II trial (SYSTEMS) of RT for pain palliation. In this study, 40 patients were recruited from three oncology centers in the UK and 35 of these patients (88%) went on to receive palliative RT (35). Five weeks after receiving 20 Gy in 5 daily fractions, 47% of the 30 assessable patients experienced an improvement in pain scores and 14/19 patients achieved either a partial response or stable disease by modified RECIST 1.1 criteria for an overall response rate of 74% at 12 weeks. These encouraging results have led the investigators to initiate the SYSTEMS-2 trial, a randomized trial of 20 Gy in 5 fractions vs. 36 Gy in 6 fractions to determine whether dose escalation might further improve pain palliation.
Future RT horizons for patients with MPM
Further improvements in the technological sophistication of the apparatus, planning systems and techniques for RT delivery along with clinical innovation will continue to afford new uses of RT in the management of patients with MPM. In addition, advances in surgical techniques along with new systemic/immunologic therapies will continue to extend survival, increasing the potential for RT-assisted durable local control to contribute to the management of these patients. The ability to place high doses of RT with precision using technologies such as SBRT allows treatment to sites of oligometastases and oligoprogression of MPM in an attempt to provide local control at multiple disease sites, although the impact this therapy on the natural history of patients with MPM still must be determined. Technologies such as image guided, stereotactic proton RT will only increase the ability to create such “oases” of local control with minimal/acceptable normal tissue damage to surrounding structures (36). In this respect, the major challenge is to better identify patients or circumstances in which this potential for improved local control might translate into changing the natural history of the disease and to conclusively demonstrate an improvement in patient survival. Finally, the ability of localized RT to activate/improve systemic host: anti-tumor immunity in conjunction with immunologic therapies such as checkpoint inhibitors is only beginning to be explored for patients with MPM (37). Thus, continued technologic, biologic and clinical innovation in RT has a tremendous potential to improve both morbidity and mortality of patients with MPM and further clinical trials are needed to determine the optimal strategies for combining advanced RT delivery techniques in the multidisciplinary care of these patients.
Acknowledgements
Funding: This work was supported in part by a grant from the National Institutes of Health P01-CA087971.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rimner A, Zauderer MG, Gomez DR, et al. Phase II Study of Hemithoracic Intensity-Modulated Pleural Radiation Therapy (IMPRINT) As Part of Lung-Sparing Multimodality Therapy in Patients With Malignant Pleural Mesothelioma. J Clin Oncol 2016;34:2761-8. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Li Y, Alley E, Friedberg J, et al. Prospective Assessment of Proton Therapy for Malignant Pleural Mesothelioma. International Association for the Study of Lung Cancer Annual Meeting, Denver, CO, 2015.
- Pan HY, Jiang S, Sutton J, et al. Early experience with intensity modulated proton therapy for lung-intact mesothelioma: A case series. Pract Radiat Oncol 2015;5:e345-53. [Crossref] [PubMed]
- Feigen M, Lee ST, Lawford C, et al. Establishing locoregional control of malignant pleural mesothelioma using high-dose radiotherapy and (18) F-FDG PET/CT scan correlation. J Med Imaging Radiat Oncol 2011;55:320-32. [Crossref] [PubMed]
- Rusch V, Baldini EH, Bueno R, et al. The role of surgical cytoreduction in the treatment of malignant pleural mesothelioma: Meeting summary of the International Mesothelioma Interest Group Congress, September 11-14, 2012, Boston, Mass. J Thorac Cardiovasc Surg 2013;145:909-10. [Crossref] [PubMed]
- Taioli E, Wolf AS, Flores RM. Meta-analysis of survival after pleurectomy decortication versus extrapleural pneumonectomy in mesothelioma. Ann Thorac Surg 2015;99:472-80. [Crossref] [PubMed]
- Yajnik S, Rosenzweig KE, Mychalczak B, et al. Hemithoracic radiation after extrapleural pneumonectomy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 2003;56:1319-26. [Crossref] [PubMed]
- Forster KM, Smythe WR, Starkschall G, et al. Intensity-modulated radiotherapy following extrapleural pneumonectomy for the treatment of malignant mesothelioma: clinical implementation. Int J Radiat Oncol Biol Phys 2003;55:606-16. [Crossref] [PubMed]
- Hill-Kayser CE, Avery S, Mesina CF, et al. Hemithoracic radiotherapy after extrapleural pneumonectomy for malignant pleural mesothelioma: a dosimetric comparison of two well-described techniques. J Thorac Oncol 2009;4:1431-7. [Crossref] [PubMed]
- Patel PR, Yoo S, Broadwater G, et al. Effect of increasing experience on dosimetric and clinical outcomes in the management of malignant pleural mesothelioma with intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 2012;83:362-8. [Crossref] [PubMed]
- Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:3007-13. [Crossref] [PubMed]
- de Perrot M, Feld R, Cho BC, et al. Trimodality therapy with induction chemotherapy followed by extrapleural pneumonectomy and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:1413-8. [Crossref] [PubMed]
- Hasegawa S, Okada M, Tanaka F, et al. Trimodality strategy for treating malignant pleural mesothelioma: results of a feasibility study of induction pemetrexed plus cisplatin followed by extrapleural pneumonectomy and postoperative hemithoracic radiation (Japan Mesothelioma Interest Group 0601 Trial). Int J Clin Oncol 2016;21:523-30. [Crossref] [PubMed]
- Gomez DR, Hong DS, Allen PK, et al. Patterns of failure, toxicity, and survival after extrapleural pneumonectomy and hemithoracic intensity-modulated radiation therapy for malignant pleural mesothelioma. J Thorac Oncol 2013;8:238-45. [Crossref] [PubMed]
- Federico R, Adolfo F, Giuseppe M, et al. Phase II trial of neoadjuvant pemetrexed plus cisplatin followed by surgery and radiation in the treatment of pleural mesothelioma. BMC Cancer 2013;13:22. [Crossref] [PubMed]
- Weder W, Stahel RA, Bernhard J, et al. Multicenter trial of neo-adjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. Ann Oncol 2007;18:1196-202. [Crossref] [PubMed]
- Van Schil PE, Baas P, Gaafar R, et al. Trimodality therapy for malignant pleural mesothelioma: results from an EORTC phase II multicentre trial. Eur Respir J 2010;36:1362-9. [Crossref] [PubMed]
- Thieke C, Nicolay NH, Sterzing F, et al. Long-term results in malignant pleural mesothelioma treated with neoadjuvant chemotherapy, extrapleural pneumonectomy and intensity-modulated radiotherapy. Radiat Oncol 2015;10:267. [Crossref] [PubMed]
- Allen AM, Czerminska M, Janne PA, et al. Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int J Radiat Oncol Biol Phys 2006;65:640-5. [Crossref] [PubMed]
- Stahel RA, Riesterer O, Xyrafas A, et al. Neoadjuvant chemotherapy and extrapleural pneumonectomy of malignant pleural mesothelioma with or without hemithoracic radiotherapy (SAKK 17/04): a randomised, international, multicentre phase 2 trial. Lancet Oncol 2015;16:1651-8. [Crossref] [PubMed]
- Rimner A, Simone CB 2nd, Zauderer MG, et al. Hemithoracic radiotherapy for mesothelioma: lack of benefit or lack of statistical power? Lancet Oncol 2016;17:e43-4. [Crossref] [PubMed]
- Lang-Lazdunski L, Bille A, Papa S, et al. Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine, prophylactic radiotherapy, and systemic chemotherapy in patients with malignant pleural mesothelioma: a 10-year experience. J Thorac Cardiovasc Surg 2015;149:558-65; discussion 565-6. [Crossref] [PubMed]
- Sugarbaker DJ, Gill RR, Yeap BY, et al. Hyperthermic intraoperative pleural cisplatin chemotherapy extends interval to recurrence and survival among low-risk patients with malignant pleural mesothelioma undergoing surgical macroscopic complete resection. J Thorac Cardiovasc Surg 2013;145:955-63. [Crossref] [PubMed]
- Friedberg JS, Simone CB 2nd, Culligan MJ, et al. Extended Pleurectomy-Decortication-Based Treatment for Advanced Stage Epithelial Mesothelioma Yielding a Median Survival of Nearly Three Years. Ann Thorac Surg 2017;103:912-9. [Crossref] [PubMed]
- Chance WW, Rice DC, Allen PK, et al. Hemithoracic intensity modulated radiation therapy after pleurectomy/decortication for malignant pleural mesothelioma: toxicity, patterns of failure, and a matched survival analysis. Int J Radiat Oncol Biol Phys 2015;91:149-56. [Crossref] [PubMed]
- Minatel E, Trovo M, Polesel J, et al. Radical pleurectomy/decortication followed by high dose of radiation therapy for malignant pleural mesothelioma. Final results with long-term follow-up. Lung Cancer 2014;83:78-82. [Crossref] [PubMed]
- de Perrot M, Feld R, Leighl NB, et al. Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2016;151:468-73. [Crossref] [PubMed]
- Boutin C, Rey F, Viallat JR. Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma. A randomized trial of local radiotherapy. Chest 1995;108:754-8. [Crossref] [PubMed]
- Bydder S, Phillips M, Joseph DJ, et al. A randomised trial of single-dose radiotherapy to prevent procedure tract metastasis by malignant mesothelioma. Br J Cancer 2004;91:9-10. [Crossref] [PubMed]
- O'Rourke N, Garcia JC, Paul J, et al. A randomised controlled trial of intervention site radiotherapy in malignant pleural mesothelioma. Radiother Oncol 2007;84:18-22. [Crossref] [PubMed]
- Bayman N, Ardron D, Ashcroft L, et al. Protocol for PIT: a phase III trial of prophylactic irradiation of tracts in patients with malignant pleural mesothelioma following invasive chest wall intervention. BMJ Open 2016;6. [Crossref] [PubMed]
- Jenkins P, Milliner R, Salmon C. Re-evaluating the role of palliative radiotherapy in malignant pleural mesothelioma. Eur J Cancer 2011;47:2143-9. [Crossref] [PubMed]
- Macleod N, Price A, O'Rourke N, et al. Radiotherapy for the treatment of pain in malignant pleural mesothelioma: a systematic review. Lung Cancer 2014;83:133-8. [Crossref] [PubMed]
- MacLeod N, Chalmers A, O'Rourke N, et al. Is Radiotherapy Useful for Treating Pain in Mesothelioma?: A Phase II Trial. J Thorac Oncol 2015;10:944-50. [Crossref] [PubMed]
- Badiyan SN, Molitoris JK, Zhu M, et al. Proton Beam Therapy for Malignant Pleural Mesothelioma. Transl Lung Cancer Res 2018;7:189-98. [Crossref] [PubMed]
- Alley EW, Katz SI, Cengel KA, et al. Immunotherapy and radiation therapy for malignant pleural mesothelioma. Transl Lung Cancer Res 2017;6:212-9. [Crossref] [PubMed]


