Atrial septal defect (ASD) device trans-catheter closure: limitations
Systematic review
Transcatheter closure is a widespread technique used to treat secundum atrial septal defects (ASDs). When compared to surgery, it provides a less invasive approach with quicker recovery and reduced physical and psychological impact (1-4).
The first case was performed in 1976 by King and Mills (5). However, the percutaneous ASD closure fully entered the clinical arena with the introduction of Amplatzer septal occluder devices (ASO) (6). Since then, many other devices have been developed and used, such as the Gore Cardioform septal occluder (GSO), the Figulla Flexible Occlutech device, the Cardioseal/Starflex and the bio absorbable devices Biostar or Biotrek (7,8).
Nowadays, almost 85–90% of all secundum ASD can be closed by using a transcatheter approach (9,10). However, several limitations may have a significant impact on the feasibility and success of percutaneous ASD closure (11,12).
Limitations can be grouped as follows: (I) anatomical limitations; (II) device-related limitations; (III) associated defects and natural history associated issues; (IV) physiological limitations; (V) complications.
Anatomical limitations
A common underlying structure apply to all available devices: they are made of two disks and a connecting segment that keeps them together across the ASD. Two different engineering concepts have been developed, so that occluder devices can be classified as self-centering and non-self-centering ones. The Amplatzer and the Amplatzer-like devices, in which a central connecting waist fills the defect improving stability and occlusion, belong to the former, while devices such as GSO, where the connecting segment is linear, belong to the latter. All the currently available devices need to have surrounding “walls” supporting their stability. In particular, the disks of non-self-centering ones should be 1.8–2 times the diameter of the defect in order to have complete defect closure and avoid mal position or embolization (8).
Main anatomical limitations to percutaneous ASD closure may be insufficient surrounding rims, multiple defects and excessively bulging atrial septal aneurisms (ASA).
Typical of ostium primum ASD and sinus venosus-type defects, deficiency of surrounding rims can affect ostium secundum ASD transcatheter closure as well. Indeed, self-centering devices need more than 5–7 mm of sufficiently stable tissue to support the device stability, while non-self-centering devices require rims 50% larger than the device length. Moreover, enough rims are necessary to avoid disks interference with atrioventricular (AV) valves and venous returns. In almost 10–15% of cases rims are too small to allow a stable and safe device implantation, so a surgical approach is chosen. However, the isolated absence of aortic rim can be overcome implanting the device so that it embraces the atrial septum around the aortic root. Occasionally rims are well represented but are too floppy and flimsy. In these cases, the tissue of the rims is too compliant and does not assure stability to the device (13-15).
Sometimes ASDs are not single but multiple. Closure is still possible, paying attention to avoid interference with both intracardiac structures and the electrical conduction system, and considering rims consistency and defect size (16,17).
Both single and multiple ASDs may be associated with ASA. ASA is defined as a localised deformity of the interatrial septum which protrudes into the right or the left atrium or both (18) and may interfere with device positioning, stability, and safety, bearing the risk of inadvertent deployment of the device with both umbrellas in the right or left atrium (19).
Because of these anatomical features, it is often necessary to perform trans oesophageal examination, cardiac catheterization and balloon sizing in order to assess the feasibility of percutaneous ASD closure. Moreover, in borderline cases, the final evaluation must be done after device implantation and release (7).
Another fundamental anatomical and physiological contraindication to ASD closure is represented by a too small ASD, with a Qp/Qs<1.5, not associated with right heart chamber enlargement nor with history of previous transient ischemic attack or stroke (20). In those cases, closure is simply not needed.
Finally, anatomical limitations may be related to the age of the patient. This is particularly true in small kids where big devices may interfere with surrounding structures. Some authors have proposed the ratio defect size (mm)/BSA as a predictor of crossover from cardiac catheterization to surgery. In particular, an ASD index >23.7 mm/m2 had a 90% specificity in “crossing over” to surgery (21). However, we think that any “index” can just give an idea of what could happen and finally an individualized analysis is mandatory.
Devices-related limitations
As previously reported, devices are currently made according to two different concepts: the self-centering and the non-self-centering idea.
ASO and Amplatzer-like devices (Figulla Flexible Occlutech septal occluder, Cocoon, CeraFlexTM ASD Occluder) belong to the self-centering group. The range of defects amenable to closure depends on the available waist diameter of the different devices. In particular, ASO waist ranges from 4 to 38 mm, Occlutech from 4 to 40 mm, Cocoon from 8 to 40 mm, and CeraFlex from 6 to 32 mm (22-26).
GSO and Helex septal occluder belong to the non-self-centering device types and the range of defect amenable to closure is up to 15–18 mm (24,27).
Associated defects and natural history associated issues
Interatrial communications can often be associated with other congenital heart defects, being crucial for survival in conditions such as hypoplastic left heart syndrome, D-transposition of great arteries, tricuspid atresia and total anomalous pulmonary venous return (27). When a surgery is needed to treat associated defects, it is logical to address surgically the concomitant secundum ASD.
Furthermore, associated defects and conditions may occur and may develop during time in long-standing ASD which causes significant right heart overload (28).
In particular, tricuspid regurgitation may develop up to a moderate or severe grade because of progressive right ventricular (RV) enlargement. It may be so severe that the isolated ASD closure will be not enough to restore tricuspid valve function, making a surgical approach necessary to both close the ASD and repair the tricuspid valve (29).
In adult subjects a concomitant coronary artery disease may develop and should be addressed (30). If surgery is needed because of coronary artery disease complexity, then percutaneous ASD closure will not be logical.
Finally, long standing ASDs are associated to the development of atrial arrhythmias, including atrial tachycardia, atrial flutter and atrial fibrillation, with an incidence of more than 10% in untreated ASDs in patients over 40 years old. Both a geometric and an electrical remodelling are responsible for the onset of such arrhythmias, the first one being characterized by atrial stretching, interstitial fibrosis, increased size of the cardiac myocytes, and ultrastructural changes, while the second one by an increased sinus node recovery time, intra-atrial conduction delay, and increased atrial effective refractory period (30). In case of known atrial arrhythmias, a comprehensive assessment, including an electrophysiologic evaluation, is mandatory in order to consider to ablate the arrhythmic substrate either percutaneously or surgically. The chosen approach will influence the technique used to close the ASD, as well (31,32).
Physiological limitations
Physiological situations where ASD closure is contra-indicated or should be performed under special circumstances include: (I) reduced compliance of the left ventricle (LV) due to cardiomyopathies or restrictive LV physiology due to aging and systemic arterial hypertension; (II) reduced compliance of the right ventricle typically in the setting of pulmonary atresia intact septum; (III) pulmonary arterial hypertension.
Reduced compliance of the left ventricle
Left ventricular compliance may decrease during life because of different causes including coronary artery disease, systemic arterial hypertension, aortic valve sclerosis. In presence of an ASD, the left-to-right shunt increases leading to pulmonary artery circulation overload, reduced LV pre-loading and de-conditioning. Abrupt ASD closure may end up in suddenly increased LV end-diastolic pressure and consequent pulmonary oedema (33-35). The transcatheter approach may help in testing contra-indication to ASD closure in such a condition: a balloon-sizing occlusion test can be performed closing the ASD for 15 minutes or for a longer time in patients with borderline coronary artery stenosis, looking for ischemic electrocardiogram (EKG) changes or regional systolic/diastolic LV abnormalities. During balloon testing, relative contra-indications to shunt closure are: (I) persistent increase of LV end-diastolic pressure (>20 mmHg and/or increase >50% compared to baseline); (II) decrease of systemic arterial pressure as higher as 20% with respect to baseline values; (III) signs of pulmonary edema as increased of post-expiratory peak pressure during mechanical ventilation or breath fatigue in awake patients (36).
In subjects with contra-indicated complete ASD closure two options are available: a drug trial with diuretics and angiotensin converting enzyme (ACE)-inhibitors for 3–6 months followed by re-evaluation of hemodynamic data during ASD balloon occlusion, or partial ASD closure using a fenestrated device (37) (Figure 1).
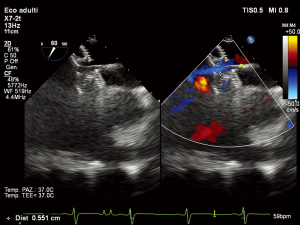
Reduced compliance of the right ventricle
RV compliance may be reduced in the setting of pulmonary atresia with intact ventricular septum (PA-IVS) septum. In subjects with PA-IVS, the long-lasting obstruction during fetal life is associated with hypertrophy and reduced diastolic properties of the RV. Furthermore, an ASD or a large patent foramen ovale (PFO) is mandatory in that setting. After surgical or transcatheter RV decompression, the atrial septal communication acts invariably as a safety valve to unload right heart and/or to increase systemic ventricular output.
However, with time, long-lasting mixed shunt at atrial level is associated with variable degree of cyanosis. Therefore, when possible, ASD closure is always needed in order to avoid right-to-left shunting caused by border-line RV compliance that is associated to central cyanosis and risks of paradoxical embolization.
Indication to ASD closure are related to clinical and echocardiographic findings. In particular, a formal contra-indication to closure is the RV unsuitability to a bi-ventricular physiology because of tricuspid valve hypoplasia (z-score <3), RV hypoplasia (bi-partite morphology or severe apical hypertrophy), significant and exclusive right-to-left atrial shunt (38).
In subjects with mild-to-moderate systemic desaturation at rest (>85%) and/or bidirectional atrial shunt at low velocity at Doppler examination, potential ASD closure should be considered. The closure suitability is evaluated during cardiac catheterization performing a sizing balloon occlusion test. The test should be performed from left atrium to avoid any interference with the right chambers volume and compliance and a dynamic balloon testing should be preferred to static ASD occlusion (Figure 2). Right atrial pressure should not increase >20%, systemic arterial pressure should not decrease >20% and oxygen saturation should increase to >94% as compared to baseline values.
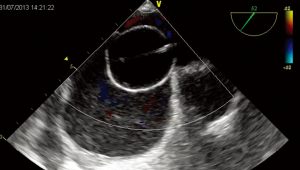
In the case of border-line changes, a short-term course of diuretics may be given to proceed a second attempt of closure some few days after or a short-term (3–6 months) trial of diuretic therapy may be set up after device deployment. In alternative, device fenestration should be considered (36).
Pulmonary arterial hypertension
Pulmonary arterial hypertension: ASDs are associated to left-to-right shunt and increased pulmonary blood flow and over time unrepaired defects may develop increased pulmonary arterial pressures. Usually resistances may remain within normal limits and defects can be closed. However, sometimes pulmonary artery resistances may increase up to 2/3 of the systemic vascular resistance and the interatrial shunt may become bidirectional or finally right-to-left, with an Eisenmenger physiology, contra-indicating the defect closure (39,40). Nevertheless, there are various intermediate situations where a partial closure with a fenestrated device that functions as a “pop-off” valve for the right atrium may be indicated (37).
Moreover, there are some subjects with ASD, pulmonary hypertension and high pulmonary vascular resistances in whom, after a course of 3–6 months of pulmonary vasodilator treatment according to a “treat and repair strategy”, ASD can be closed thanks to a substantial reduction of vascular resistance levels (41).
Complications
Complications may limit ASD transcatheter closure feasibility and success. They include device embolization, new complete AV block (CAVB) onset and myocardial erosion (42,43).
Device embolization may occur in up to 1% of cases. The commonest reasons for occluder dislodgement are the use of an undersized ASD device, greater defect size, left atrium too small to accommodate the device, an inadequate or floppy rim, device mobility post-implantation, and operator-related technical issues (44). The most of dislodgement occurs within 24 hours post implantation and takes place into left atrium (24.6%), aorta (18.4%), and right ventricle (16.7%) (45,46). The majority of the devices can be retrieved percutaneously after early embolization. In such cases the defect is re-evaluated and, if a misevaluation or misplacement of the device occurred during the first procedure, a correct second procedure may end up in success (47,48). On the other hand, late embolization usually requires surgical treatment because of epithelization (49).
CAVB is reported to occur sporadically at the time of the procedure or hours and days later, requiring initial steroid therapy and, in case of no responsiveness, subsequent surgical removal of the device and closure of the ASD. It is generally thought to be caused by compression of the AV node as well as by inflammatory foreign body reaction or scarring at the Koch’s triangle level due to the device presence. A single case of compression of a small coronary artery providing alternative blood supply to the AV node has been described (50). Risk factors for CAVB after device ASD closure are considered deficient postero-inferior rims, the use of large device in small children, and previous AV node conduction disturbances. Even if CAVB can resolve permanently, sometimes percutaneous or epicardial pacemaker implantation is necessary (50-53). The most feared complication is cardiac erosion (54). Its incidence has been estimated to range between 0.1% and 0.3%. Usually cardiac erosion occurs early after the procedure, with most cases developing within 1 year of device closure. However, some reports describe cardiac erosion occurring later (55). Wall perforation usually involves the roof of the right or left atrium or of the atrial junction with the aorta (Figure 3), causing hemopericardium, tamponade or aortic fistula (56). Patients generally show chest pain, dizziness and pericardial tamponade symptoms and require emergency cardiac surgery to explant the device and repair the perforation (57). Risk factors are not completely understood, but deficient anterior and/or superior rims, device movement in the heart, oversized devices, adult age, occluder type (ASO, Occlutech occluder and Cardia devices) and multiple attempts at deployment of the device prior to the final delivery, have been postulated to be related to cardiac erosion (58,59).
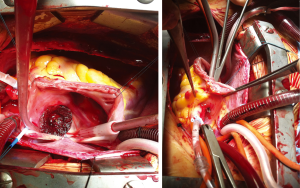
Conclusions
Transcatheter ASD closure is a widespread procedure. However, several anatomical, device-related and physiological issues may limit its results and feasibility. Furthermore, associated defects or the occurrence of complications represent known limitations.
Notwithstanding that, percutaneous ostium secundum ASD closure remains the first line therapy for the treatment of these defects.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ooi YK, Kelleman M, Ehrlich A. Transcatheter Versus Surgical Closure of Atrial Septal Defects in Children: A Value Comparison. JACC Cardiovasc Interv 2016;9:79-86. [Crossref] [PubMed]
- Villablanca PA, Briston DA, Rodés-Cabau J, et al. Treatment options for the closure of secundum atrial septal defects: A systematic review and meta-analysis. Int J Cardiol 2017;241:149-55. [Crossref] [PubMed]
- Butera G, Biondi-Zoccai G, Sangiorgi G, et al. Percutaneous versus surgical closure of secundum atrial septal defects: a systematic review and meta-analysis of currently available clinical evidence. EuroIntervention 2011;7:377-85. [Crossref] [PubMed]
- Butera G, Carminati M, Chessa M, et al. Percutaneous versus surgical closure of secundum atrial septal defect: comparison of early results and complications. Am Heart J 2006;151:228-34. [Crossref] [PubMed]
- King TD, Mills NL. Secundum atrial septal defects: non-operative closure during cardiac catheterisation. JAMA 1976;235:2506-9. [Crossref] [PubMed]
- Masura J, Gavora P, Formanek A, et al. Transcatheter closure of secundum atrial septal defects using the new self-centering Amplatzer septal occluder: initial human experience. Cathet Cardiovasc Diagn 1997;42:388-93. [Crossref] [PubMed]
- Kazmouz S, Kenny D, Cao QL, et al. Transcatheter closure of secundum atrial septal defects. J Invasive Cardiol 2013;25:257-64. [PubMed]
- Nassif M, Abdelghani M, Bouma BJ, et al. Historical developments of atrial septal defect closure devices: what we learn from the past. Expert Rev Med Devices 2016;13:555-68. [Crossref] [PubMed]
- Butera G, De Rosa G, Chessa M, et al. Transcatheter closure of atrial septal defect in young children: results and follow-up. J Am Coll Cardiol 2003;42:241-5. [Crossref] [PubMed]
- Butera G, Romagnoli E, Carminati M, et al. Treatment of isolated secundum atrial septal defects: impact of age and defect morphology in 1,013 consecutive patients. Am Heart J 2008;156:706-12. [Crossref] [PubMed]
- Baruteau AE, Petit J, Lambert V, et al. Transcatheter closure of large atrial septal defects: feasibility and safety in a large adult and pediatric population. Circ Cardiovasc Interv 2014;7:837-43. [Crossref] [PubMed]
- Meyer MR, Kurz DJ, Bernheim AM, et al. Efficacy and safety of transcatheter closure in adults with large or small atrial septal defects. Springerplus 2016;5:1841. [Crossref] [PubMed]
- Kijima Y, Akagi T, Takaya Y, et al. Deficient Surrounding Rims in Patients Undergoing Transcatheter Atrial Septal Defect Closure. J Am Soc Echocardiogr 2016;29:768-76. [Crossref] [PubMed]
- Oflaz MB, Pac FA, Kibar AE, et al. Evaluation of morphological characteristics of septal rims affecting successful transcatheter atrial septal defect closure in children and adults. Postepy Kardiol Interwencyjnej 2013;9:205-11. [Crossref] [PubMed]
- O'Byrne ML, Gillespie MJ, Kennedy KF, et al. The influence of deficient retro-aortic rim on technical success and early adverse events following device closure of secundum atrial septal defects: An Analysis of the IMPACT Registry®. Catheter Cardiovasc Interv 2017;89:102-11. [Crossref] [PubMed]
- Yang Y, Xu Z, Jiang S, et al. Simultaneous Transcatheter Closure of Multiple Atrial Septal Defects Using Dual Amplatzer Septal Occluder Devices. Am J Med Sci 2016;352:245-51. [Crossref] [PubMed]
- Butera G, Romagnoli E, Saliba Z, et al. Percutaneous closure of multiple defects of the atrial septum: procedural results and long-term follow-up. Catheter Cardiovasc Interv 2010;76:121-8. [Crossref] [PubMed]
- Gallet B, Malergue MC, Adams C, et al. Atrial septal aneurysm--a potential cause of systemic embolism. An echocardiographic study. Br Heart J 1985;53:292-7. [Crossref] [PubMed]
- Peuster M, Kaulitz R, Hausdorf G. A novel method for transcatheter closure of atrial septal defect within an aneurysm of the fossa ovalis: double sheath technique. Heart 2000;84. [Crossref] [PubMed]
- Baumgartner H, Bonhoeffer P, De Groot NM, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915-57. [Crossref] [PubMed]
- Mulukutla V, Qureshi AM, Pignatelli R, et al. Predictive Factors for Patients Undergoing ASD Device Occlusion Who "Crossover" to Surgery. Pediatr Cardiol 2018;39:445-9. [Crossref] [PubMed]
- Bissessor N. Current perspectives in percutaneous atrial septal defect closure devices. Med Devices (Auckl) 2015;8:297-303. [Crossref] [PubMed]
- Thanopoulos BD, Biasco L, Dardas P, et al. Catheter closure of atrial septal defects using the Cocoon septal occluder: preliminary results of a European multicenter study. Int J Cardiol 2014;177:418-22. [Crossref] [PubMed]
- Tang B, Su F, Sun X, et al. Recent development of transcatheter closure of atrial septal defect and patent foramen ovale with occluders. J Biomed Mater Res B Appl Biomater 2018;106:433-43. [Crossref] [PubMed]
- Apostolopoulou SC, Tsoutsinos A, Laskari C, et al. Large single centre experience with the Cera™ and CeraFlex™ occluders for closure of interatrial communications: usefulness of the flexible rotation feature. Cardiovasc Interv Ther 2018;33:70-6. [Crossref] [PubMed]
- Haas NA, Soetemann DB, Ates I, et al. Closure of Secundum Atrial Septal Defects by Using the Occlutech Occluder Devices in More Than 1300 Patients: The IRFACODE Project: A Retrospective Case Series. Catheter Cardiovasc Interv 2016;88:571-81. [Crossref] [PubMed]
- Grohmann J, Höhn R, Fleck T, et al. Transcatheter closure of atrial septal defects in children and adolescents: single-center experience with the GORE® septal occluder. Catheter Cardiovasc Interv 2014;84:E51-7. [Crossref] [PubMed]
- Sachdeva R. Atrial Septal Defect. In Moss and Adams’ Heart disease in infants, children and adolescents. 9th edition. Wolters Kluwer, 2016:739-802.
- Chen L, Shen J, Shan X, et al. Improvement of tricuspid regurgitation after transcatheter ASD closure in older patients. Herz 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Authors/Task Force members, Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541-619. [Crossref] [PubMed]
- Contractor T, Mandapati R. Arrhythmias in Patients with Atrial Defects. Card Electrophysiol Clin 2017;9:235-44. [Crossref] [PubMed]
- Giamberti A, Pluchinotta FR, Chessa M, et al. Surgery for supraventricular tachycardia and congenital heart defects: long-term efficacy of the combined approach in adult patients. Europace 2017;19:1542-8. [PubMed]
- Ewert P, Berger F, Nagdyman N, et al. Masked left ventricular restriction in elderly patients with atrial septal defects: a contraindication for closure? Catheter Cardiovasc Interv 2001;52:177-80. [Crossref] [PubMed]
- Tashiro H, Suda K, Iemura M, et al. Intergenerational differences in the effects of transcatheter closure of atrial septal defects on cardiac function. J Cardiol 2017;70:620-6. [Crossref] [PubMed]
- Chigurupati K, Reshmi LJ, Gadhinglajkar S, et al. Pulmonary edema following transcatheter closure of atrial septal defect. Ann Card Anaesth 2015;18:441-4. [Crossref] [PubMed]
- Vasquez AF, Lasala JM. Atrial septal defect closure. Cardiol Clin 2013;31:385-400. [Crossref] [PubMed]
- Abdelkarim A, Levi DS, Tran B, et al. Fenestrated Transcatheter ASD Closure in Adults with Diastolic Dysfunction and/or Pulmonary Hypertension: Case Series and Review of the Literature. Congenit Heart Dis 2016;11:663-71. [Crossref] [PubMed]
- Alwi M. Management Algorithm in Pulmonary Atresia with Intact Ventricular Septum. Catheter Cardiovasc Interv 2006;67:679-86. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119. [Crossref] [PubMed]
- El-Kersh K, Howsare M, Zaidi A, et al. Pulmonary Hypertension and Atrial Septal Defect: Management Dilemma. Am J Ther 2017;24:e602-5. [Crossref] [PubMed]
- Akagi S, Nakamura K, Akagi T, et al. Feasibility of Repairing Defects Followed by Treatment with Pulmonary Hypertension-specific Drugs (Repair and Treat) in Patients with Pulmonary Hypertension Associated with Atrial Septal Defect: Study Protocol for Interventional Trial. Acta Med Okayama 2016;70:397-400. [PubMed]
- Moore J, Hegde S, El-Said H, et al. Transcatheter device closure of atrial septal defects: a safety review. JACC Cardiovasc Interv 2013;6:433-42. [Crossref] [PubMed]
- Chessa M, Carminati M, Butera G, et al. Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol 2002;39:1061-5. [Crossref] [PubMed]
- Martínez-Quintana E, Rodríguez-González F. Risks Factors for Atrial Septal Defect Occlusion Device Migration. Int J Angiol 2016;25:e63-5. [PubMed]
- Lee WC, Fang CY, Huang CF, et al. Predictors of Atrial Septal Defect Occluder Dislodgement. Int Heart J 2015;56:428-31. [Crossref] [PubMed]
- Deşer SB, Demirağ MK. Migration of an Atrial Septal Occluder Device with Formation of Abdominal Aortic Dissection. Ann Thorac Surg 2017;103:e343-4. [Crossref] [PubMed]
- Katta N, Gautam S, Webel R. Successful Percutaneous Retrieval of Embolized Septal Occluder Device from Aortic Arch and Placement of a Newer Septal Occluder Device in Combined Procedure. Case Rep Cardiol 2016;2016. [Crossref] [PubMed]
- Shebani SO, Rehman R, Taliotis D, et al. Techniques for transcatheter retrieval of the occlutech ASD device United Kingdom-European multicenter report. Catheter Cardiovasc Interv 2017;89:690-8. [Crossref] [PubMed]
- Yates MT, Anderson DR. Safe Surgical Retrieval of Embolized Atrial Septal Defect Closure Device. Ann Thorac Surg 2017;103:e213-4. [Crossref] [PubMed]
- Yamamoto T, Kanazawa H, Tanosaki S, et al. A Novel Mechanism of Atrioventricular Block Following Transcatheter Closure of an Atrial Septal Defect. JACC Cardiovasc Interv 2016;9:2067-9. [Crossref] [PubMed]
- Asakai H, Weskamp S, Eastaugh L, et al. Atrioventricular block after ASD closure. Heart Asia 2016;8:26-31. [Crossref] [PubMed]
- Dittrich S, Sigler M, Priessmann H. Late complete atrioventricular block after closure of an atrial septal defect with a gore septal occluder (GSO™). Catheter Cardiovasc Interv 2016;87:945-50. [Crossref] [PubMed]
- Al-Anani SJ, Weber H, Hijazi ZM. Atrioventricular block after transcatheter ASD closure using the Amplatzer septal occluder: risk factors and recommendations. Catheter Cardiovasc Interv 2010;75:767-72. [Crossref] [PubMed]
- Mitchelson B, O'Donnell C, Ruygrok P, et al. Transcatheter closure of secundum atrial septal defects: has fear of device erosion altered outcomes? Cardiol Young 2017;27:1153-61. [Crossref] [PubMed]
- Kim JS, Yeom SY, Kim SH, et al. Delayed Left Atrial Perforation Associated with Erosion After Device Closure of an Atrial Septal Defect. Korean J Thorac Cardiovasc Surg 2017;50:110-3. [Crossref] [PubMed]
- Crawford GB, Brindis RG, Krucoff MW, et al. Percutaneous atrial septal occluder devices and cardiac erosion: a review of the literature. Catheter Cardiovasc Interv 2012;80:157-67. [Crossref] [PubMed]
- Arnaz A, Turkekul Y, Yalcinbas Y, et al. Late Cardiac Rupture after Amplatzer Septal Occluder Implantation. Tex Heart Inst J 2016;43:541-2. [Crossref] [PubMed]
- Thomson JD, Qureshi SA. Device closure of secundum atrial septal defect's and the risk of cardiac erosion. Echo Res Pract 2015;2:R73-8. [Crossref] [PubMed]
- McElhinney DB, Quartermain MD, Kenny D, et al. Relative Risk Factors for Cardiac Erosion Following Transcatheter Closure of Atrial Septal Defects: A Case-Control Study. Circulation 2016;133:1738-46. [Crossref] [PubMed]


