Scientific statement on spontaneous coronary artery dissection: care must be taken not to miss the association of spontaneous coronary artery dissection and takotsubo syndrome
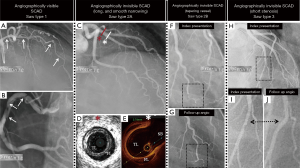
Spontaneous coronary artery dissection (SCAD) is defined as a non-traumatic, non-iatrogenic, and non-atherosclerotic acute spontaneous separation of the coronary arterial wall layers due to bleeding into the arterial wall resulting in a false lumen compressing the true lumen and compromising the coronary arterial flow. The invasive coronary angiography (CAG) is the most important diagnostic route to identify SCAD. According to the findings of CAG, SCAD is classified simply into angiographically visible SCAD [type1 according to Saw classification (1)] and angiographically invisible SCAD [type 2A, 2B and type 3 according to Saw classification (1)]. In the angiographically visible SCAD, the pathognomonic angiographic signs as double or multiple radiolucent lumens of different opacities, radiolucent intimal flap, or contrast staining of the vessel wall are seen during CAG (Figure 1A,B); in detecting these signs, the diagnosis of SCAD will be confirmed. In the angiographically invisible SCAD, the above-mentioned pathognomonic signs are not seen. In the invisible SCAD, there will be usually a long diffuse smooth narrowing of the coronary artery with an abrupt demarcation of the proximal normal part of the vessel and a normal segment after the end of the lesion (type 2A) (Figure 1C,D,E). The invisible SCAD may involve the peripheral segments of the coronary arteries and seen as “normal tapering” vessel (type 2B) (Figure 1F,G). The SCAD lesion in the invisible SCAD may also be short mimicking an atherosclerotic coronary lesion (type 3) (Figure 1H,I,J). With invasive CAG, the diagnosis of invisible SCAD will be only suspected and may be confirmed by invasive intra-coronary imaging as intravascular ultrasound (IVUS) imaging and optical coherence tomography (OCT) imaging (2-4) (Figure 1D,E), where an intimal tear and/or an intramural hematoma and double lumen (false and true) will confirm the SCAD diagnosis. When the SCAD lesion is distal and the intravascular imaging deemed to be associated with substantial risks, the diagnosis of the invisible SCAD may be confirmed with repeated CAG after 6–8 weeks where angiographic healing of the SCAD lesions is seen (Figure 1G,J). Computed cardiac tomography angiography (CCTA) may also be valuable for noninvasive follow up of patients with proximal SCAD. SCAD involving the distal or small coronary arteries is generally not visualized with CCTA.
Recently, an expert group published an outstanding scientific statement from the American Heart Association (AHA) on SCAD: current state on science (5). This scientific statement provides an overview of the current evidence and expert consensus on the management of the disease. In this statement, it is noticed that there is a remarkable evolution of our knowledge on the subject during the last 5 to 8 years (5). The disease is far more common than previously thought and affects higher older than previously reported. This is mainly due to detection of angiographically invisible SCAD affecting mainly the mid-distal segments of the coronary arteries, which previously were missed or misdiagnosed (1,5). In the contemporary studies (6,7) the pregnancy-related SCAD (P-SCAD) represents approximately 5% to 10% of SCAD cases compared to 30% in the previous reports (8). Worth to mention, P-SCAD has poorer prognosis compared to women with SCAD unrelated to pregnancy because of increased involvement of left main stem, proximal coronary segments and multi-vessel involvement. Because of these potential risks, many clinicians recommend against subsequent pregnancy in women with a history of SCAD (5,6). High prevalence of fibro-muscular dysplasia (FMD) in other vascular beds and a strong association between FMD and SCAD has been detected during the last 6 years (7,9). High prevalence of coronary artery tortuosity as a predisposing factor for SCAD has also been observed (5). Conservative management is the recommended treatment strategy in SCAD because of spontaneous healing of the dissected vessel wall and the percutaneous coronary intervention (PCI) in patients with SCAD is associated with higher technical failure and complication rates (6,7). Consequently, treatment of SCAD differs from that of atherosclerotic-caused acute coronary syndrome (ACS) (5). Despite the remarkable evolution of our knowledge on the disease, SCAD is still under-diagnosed (5,6). Different treatment strategies and the effectivity, safety and duration of antithrombotic and anti-platelet therapy need to be exposed in randomized controlled studies (5,6). Regrettably, one point was lacking in this excellent scientific statement and that is the view of the expert group on the association of SCAD and takotsubo syndrome (5).
Detection of the angiographically invisible SCAD
The wide-spread use of invasive CAG in patients with ACS, the utilization of intravascular imaging such as IVUS and OCT, and the increased awareness of the physicians particularly the coronary angiographers have resulted in the detection and recognition of the angiographically invisible SCAD during the last 5 to 10 years (1), which almost always were previously missed or misdiagnosed. It has been shown that the angiographically invisible SCAD constitute about 70% of patients with SCAD (7). Consequently, the disease is far more common than previously thought; SCAD may be a cause of up to 1% to 4% of ACS (5). The disease affects higher age than previously reported. Before 2011, the mean age reported at presentation was 30 to 45 years compared to the mean age in contemporary series ranging from 44 to 53 years (8). This is mainly due to detection of SCAD cases in middle aged patients, which were previously missed. However, despite the tremendous improvements in our knowledge on the diagnosis and detection of the disease, SCAD continues to be missed, misdiagnosed and consequently mismanaged. For this reason, it is important for the angiographers to recognize some angiographic features of angiographically invisible SCAD. The lesions are usually long, diffuse with smooth narrowing and may have abrupt demarcation at the beginning and the end of SCAD lesion. Because SCAD lesion affects predominantly the mid-distal segments of the coronary arteries, some lesion may be seen as normal tapering vessel (5). The distal segments of the coronary arteries should be critically reviewed. Some SCAD lesions have been described as “stick insect appearance” (6) caused by an extrinsic compression of the intramural hematoma, with biconcave aspect of the lumen, often interrupted by a side branch. SCAD lesions have also been described as having “raddish appearance” (6) caused by extrinsic hematoma compression resulting in distal occlusion. The intramural lumen may fill and empty slowly and may distally end in a “cul de sac” (8) with stasis of dye in between the injections. The SCAD lesions have also been described as long interrupted lesion or “broken line”. Further findings, which may strengthen the suspicion of SCAD are a coronary lesion in young female patients with absence of conventional cardiovascular risk factors and normal remainder coronary arteries (7).
Fibromuscular dysplasia (FMD) as a predisposing arteriopathy for SCAD
FMD is a non-inflammatory, non-atherosclerotic disease of the arterial wall. It occurs predominantly in middle aged females with few cardiovascular risk factors (6,9). FMD may manifest as arterial tortuosity, stenosis, aneurysm, and dissection in medium-sized arteries. SCAD may be a manifestation of FMD in coronary arteries also (7,9). FMD has been reported in 17–86% of screened patients with SCAD (6,7). This high prevalence of FMD in SCAD patients has been detected during the last 5–8 years (9). As late as 2010, in the excellent review article by Vrints (8), the term FMD as a predisposing factor for SCAD was not mentioned. Owing to the high prevalence of FMD in patients with SCAD, it will be persuasive to speculate that the SCAD is a complication of underlying coronary FMD in a substantial number of patients. The first case series reported on the association of SCAD and extra-coronary FMD was in 2012 (9). Interestingly, Pate et al. (10) in 2005 described 7 women presented with ACS with coronary lesions typical for angiographically invisible SCAD but deemed as changes attributed to coronary FMD because all 7 patients had FMD in the renal arteries. SCAD patients with coronary tortuosity have more frequently extra-coronary FMD. Coronary tortuosity was also shown to be a coronary manifestation of FMD (5,6). In one study (11), severe coronary tortuosity had a borderline association with higher risk of recurrent SCAD with recurrence most likely to occur in the tortuous segments. FMD may increase the risk of recurrence in SCAD; 32 of 43 (74.4%) patients with recurrent SCAD had FMD (12). In SCAD patients, screening for the SCAD associated extra-coronary arteriopathy with vascular imaging from brain to pelvis should be considered. In patients undergoing CAG to diagnose SCAD, additional non-selective or selective renal and iliac artery catheter-based angiography can be done in the same setting to assess for FMD (5). For the detection extra- or intra-cranial signs of FMD and its complications non-invasive CCTA is recommended (5).
The association of SCAD and takotsubo syndrome
SCAD and TS have 3 important features in common; both affect predominantly women; in both conditions, the disease may be preceded by an emotional or an extreme physical stress factor; and “restitution ad integrum” is the rule in SCAD with angiographic healing of the dissected vessel and also in TS with recovery of left ventricular dysfunction within weeks (7,13). Intense emotional stress (usually in women) and an extreme physical exercise (usually in men) are the most commonly reported precipitants of SCAD (5). Although the authors of the AHA scientific statement on SCAD have acknowledged that “a similar mechanism was proposed in other stress-induced cardiovascular conditions such as stress-induced “takotsubo cardiomyopathy (takotsubo syndrome)”, the investigators have concluded that “Care must be taken to ensure that SCAD is not misinterpreted as among others takotsubo syndrome”. Interestingly, the recently published ESC position paper on SCAD (6) has not either mentioned anything on the association of SCAD and TS; they have only referred to “takotsubo cardiomyopathy” as one of the differential diagnosis of SCAD. Nevertheless, if an intense emotional stressor or an extreme physical exercise may trigger SCAD or TS, it will be intuitive to conclude that such extreme stress factors may induce both SCAD and TS in the same patient concurrently (14). Additionally, sufficient evidence supporting the notion that ACS in general and SCAD in particular triggering TS are found in the literature including those that are published by the co-authors of the AHA scientific statement on SCAD (14-18). During the last few years, tens of cases of ACS including several cases of SCAD inducing TS have been reported (14,16-18). The most plausible mechanism of SCAD inducing TS is through the acute ischemic insult, the chest pain and all the discomfort caused by the ACS, which may act as an intense physical stressor. The reported cases of SCAD inducing TS have revealed on cardiac magnetic resonance (CMR) imaging a limited myocardial infarction matching the dissected vessel (16,18). Simultaneously, these patients had left ventricular wall motion abnormality (LVWMA) extending beyond the infarcted segments and beyond the areas subtended by the dissected coronary artery (16,18). There will be no objection to deem these LVWMAs as post ischemic myocardial stunning (PIMS). Furthermore, the CMR imaging of these PIMS regions have circumferential pattern with edema but no late gadolinium enhancement (LGE) (18). In addition, this LVWMA caused by myocardial stunning is completely reversible. Those features seen in PIMS are typical for that of TS. Consequently, PIMS is a form of TS induced by an acute ischemic insult. These findings are well-illustrated in Figure 2. Further support for this hypothesis is provided elsewhere (14,19). Chao et al. (20) reported apical or mid-apical ballooning typical for TS in 26% of patients with LAD ST-elevation myocardial infarction. Recently, Iwaszczuk et al. (21), reported on 120 patients presented with clinical ACS. Ninety-one patients (75.8%) had ischemic LVWMA, 21 (17.5%) had ischemic LVWMA and LVWMA, which extended beyond the diseased vessel and the authors appropriately used the term neurogenic LVWMA, and 8 (6.7%) had only neurogenic LVWMA. The last group with only neurogenic LVWMA had most probably TS. Among the 112 patients with ischemic group, 21 (18.8%) had both ischemic LVWMA and neurogenic LVWMA, which can reasonably be interpreted as ACS-induced TS.
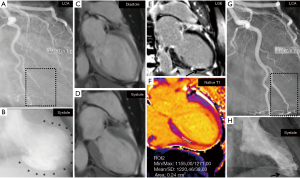
Co-authors of the AHA scientific statement on SCAD have highlighted on the importance of assessing the LVWMA in patients with angiographically invisible SCAD as this “telltale sign” was present in most of those patients (22). Franco et al. (23) reported on LVWMA and the changes in left ventricular function in hitherto the largest number of patients with SCAD. In 277 patients with SCAD, 237 patients (85.6%) had LVWMA, which according to the authors corresponded to the arterial distribution of their SCAD. In 72 of 277 patients (26%), the left ventricular ejection fraction (LVEF) was <50%, the remainder had LVEF >50%. Interestingly, 92.4% of patients underwent contrast left ventriculography during CAG. Repeat assessment of left ventricular function (164 patients) was done predominantly by contrast left ventriculography (50%) and echocardiography (46.3%). Baseline LVEF of <50% was observed in 29.9%, but in only 6.7% had LVEF <50% at follow-up. The authors deemed appropriately that the improvement in left ventricular function may reflect resolution of myocardial stunning after spontaneous healing of the SCAD lesions. Consequently, the authors hypothesized that the SCAD can result in prolonged myocardial ischemia in the territory subtended the dissected coronary artery, potentially causing myocardial stunning to a greater degree than myocardial infarction, which may consequently lead to improvement of left ventricular function with vessel healing. However, many details, which could have answered the disagreement on the association of SCAD and TS, are missing in that study. Details on the distribution and pattern of myocardial stunning are lacking. Figures on left ventricular silhouette during systole are highly desirable but regrettably lacking. CMR imaging could have contributed with invaluable information on the extent of both myocardial infarction and myocardial stunning but unavailable. However, the authors have acknowledged that in some patients with SCAD the myocardial stunning extended beyond the infarcted region. Although the authors stated that the LVWMA corresponded to the dissected vessel, the LVWMA in their previous report on “SCAD misdiagnosed as TS” in 9 patients (15) extended absolutely beyond the dissected vessel and had features consistent with TS and were in fact diagnosed as TS by the treating physicians (24). A diagonal SCAD causing a limited myocardial infarction in the left ventricular wall concurrently with reversible LVWMA involving the anterolateral, apical and inferior wall (mid-apical ballooning) is absolutely a case of SCAD associated with TS and not SCAD misdiagnosed as TS (15,16). This conclusion has been supported by other investigators (25). The association of SCAD and TS may have important implications on the management of patients. Severe hypotension in such patients may be interpreted as cardiogenic shock and treated with inotropic medications and interventional revascularization due to suspicion of SCAD progression. However, such severe hypotension in SCAD associated with TS may be due to left ventricular outflow tract obstruction where the treatment is completely different. I would rather conclude that care must be taken to ensure that not to miss the association of SCAD and TS. I strongly recommend the authors of AHA excellent scientific statement on SCAD and even the ESC statement position on SCAD to reconsider their view on the subject in details.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv 2014;84:1115-22. [Crossref] [PubMed]
- Alfonso F, Paulo M, Gonzalo N, et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol 2012;59:1073-9. [Crossref] [PubMed]
- Franco C, Eng L, Saw J. Optical Coherence Tomography in the Diagnosis and Management of Spontaneous Coronary Artery Dissection. Interv Cardiol Clin 2015;4:309-20. [Crossref] [PubMed]
- Buccheri D, Milazzo D, Geraci S, et al. A lesson from intravascular imaging: insights for recognizing a spontaneous coronary artery dissection. J Thorac Dis 2017;9:5363-7. [Crossref] [PubMed]
- Hayes SN, Kim ESH, Saw J, et al. Spontaneous Coronary Artery Dissection: Current State of the Science: A Scientific Statement From the American Heart Association. Circulation 2018;137:e523-57. [Crossref] [PubMed]
- Adlam D, Alfonso F, Maas A, et al. European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Saw J, Mancini GB, Humphries KH. Contemporary Review on Spontaneous Coronary Artery Dissection. J Am Coll Cardiol 2016;68:297-312. [Crossref] [PubMed]
- Vrints CJ. Spontaneous coronary artery dissection. Heart 2010;96:801-8. [Crossref] [PubMed]
- Saw J, Poulter R, Fung A, et al. Spontaneous coronary artery dissection in patients with fibromuscular dysplasia: a case series. Circ Cardiovasc Interv 2012;5:134-7. [Crossref] [PubMed]
- Pate GE, Lowe R, Buller CE. Fibromuscular dysplasia of the coronary and renal arteries? Catheter Cardiovasc Interv 2005;64:138-45. [Crossref] [PubMed]
- Eleid MF, Guddeti RR, Tweet MS, et al. Coronary artery tortuosity in spontaneous coronary artery dissection: angiographic characteristics and clinical implications. Circ Cardiovasc Interv 2014;7:656-62. [Crossref] [PubMed]
- Main A, Prakash R, Starovoytov A, et al. Characteristics of extension and de novo recurrent spontaneous coronary artery dissection. EuroIntervention 2017;13:e1454-9. [Crossref] [PubMed]
- Y-Hassan S. Tornvall P. Epidemiology, pathogenesis, and management of takotsubo syndrome. Clin Auton Res 2018;28:53-65. [Crossref] [PubMed]
- Y-Hassan S. Spontaneous coronary artery dissection and takotsubo syndrome: An often overlooked association Cardiovasc Revasc Med 2018. review.
- Chou AY, Sedlak T, Aymong E, et al. Spontaneous Coronary Artery Dissection Misdiagnosed as Takotsubo Cardiomyopathy: A Case Series. Can J Cardiol 2015;31:1073.e5-8. [Crossref] [PubMed]
- Y-Hassan S. Bohm F. The causal link between spontaneous coronary artery dissection and takotsubo syndrome: A case presented with both conditions. Int J Cardiol 2016;203:828-31. [Crossref] [PubMed]
- Messas N, Blondet C, Jesel L, et al. Diagnostic relevance of optical coherence tomography imaging in aborted acute myocardial infarction with a "Takotsubo component". Int J Cardiol 2015;195:123-5. [Crossref] [PubMed]
- Y-Hassan S. Themudo R, Maret E. Spontaneous coronary artery dissection and takotsubo syndrome: The chicken or the egg causality dilemma. Catheter Cardiovasc Interv 2017;89:1215-8. [Crossref] [PubMed]
- Y-Hassan S. Post-ischemic myocardial stunning was the starting point of takotsubo syndrome: Restitution is justified after falling down on. Int J Cardiol 2015;198:174-5. [Crossref] [PubMed]
- Chao T, Lindsay J, Collins S, et al. Can acute occlusion of the left anterior descending coronary artery produce a typical "takotsubo" left ventricular contraction pattern? Am J Cardiol 2009;104:202-4. [Crossref] [PubMed]
- Iwaszczuk P, Kolodziejczyk B, Kruczek T, et al. Ischemic Versus Non-Ischemic (Neurogenic) Myocardial Contractility Impairment in Acute Coronary Syndromes: Prevalence and Impact on Left Ventricular Systolic Function Recovery. Med Sci Monit 2018;24:3693-701. [Crossref] [PubMed]
- Saw J, Mancini GB, Humphries K, et al. Angiographic appearance of spontaneous coronary artery dissection with intramural hematoma proven on intracoronary imaging. Catheter Cardiovasc Interv 2016;87:E54-61. [Crossref] [PubMed]
- Franco C, Starovoytov A, Heydari M, et al. Changes in left ventricular function after spontaneous coronary artery dissection. Clin Cardiol 2017;40:149-54. [Crossref] [PubMed]
- Y-Hassan S. Takotsubo Syndrome in Patients With Spontaneous Coronary Artery Dissection. Misdiagnosis or a Reality? Can J Cardiol 2015;31. [Crossref] [PubMed]
- Buccheri D, Zambelli G. The link between spontaneous coronary artery dissection and takotsubo cardiomyopathy: analysis of the published cases. J Thorac Dis 2017;9:5489-92. [Crossref] [PubMed]


