Effect of cyclosporine A on mortality after acute exacerbation of idiopathic pulmonary fibrosis
Introduction
Acute exacerbation of idiopathic pulmonary fibrosis (AE-IPF) is defined as acute, clinically significant respiratory deterioration, characterised by evidence of new widespread alveolar abnormalities (1). The reported incidence of AE-IPF was 41 per 1,000 patient-years (2), and the AE-IPF-associated mortality was high (55.6–80%) (3-6).
There is currently no recognized treatment for AE-IPF, though international evidence-based guidelines weakly recommend standard therapy involving systemic glucocorticoids, including intravenous methylprednisolone 1 g/day for 3 days (7).
Cyclosporine A is an immunosuppressant that binds to and inhibits calcineurin, restricting lymphocyte proliferation by downregulating transcription of interleukin-2 and other cytokines associated with T helper lymphocytes (8). Methylprednisolone plus cyclosporine A has been used for AE-IPF patients in real-world clinical settings; however, the above guidelines do not comment on this use of cyclosporine A for AE-IPF because of a lack of evidence (7,9-11). Although some case series existed, the effects of cyclosporine A in AE-IPF patients thus currently remain unknown (9-11).
The present study aimed to compare the effectiveness of cyclosporine A combined with systemic glucocorticoids with systemic glucocorticoids alone for reducing mortality in patients with AE-IPF, using data from a national inpatient database in Japan.
Methods
Data source
Inpatient data were extracted from the Japanese Diagnosis Procedure Combination database. More than 1,000 hospitals voluntarily contribute to the database, which includes data on approximately 7 million inpatients, representing approximately 50% of all discharges from acute care hospitals in Japan. The data used in the present study included hospital identification numbers; ZIP codes for patient residence; patient sex and age; body weight and height; consciousness level on admission; dates of hospitalization and discharge; main diagnoses, pre-existing comorbidities on admission, and complications that occurred during hospitalization recoded with the International Classification of Diseases, tenth revision (ICD-10) codes and text in Japanese; surgical and nonsurgical procedures and dates of the procedures performed; dates and doses of drugs or blood products administered during the hospitalization; and discharge status.
The Institutional Review Board of The University of Tokyo approved this study. Informed consent was waived because of the anonymous nature of the data.
Patient selection
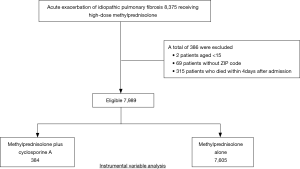
This study used data from July 1, 2010, to March 31, 2014. The inclusion criteria were patients aged ≥15 years who were diagnosed with IPF (ICD-10 codes: J84.1, J84.8, and J84.9) who received computed tomography within 1 day after admission, and who did not receive furosemide infusion within 1 day after admission (1). We excluded patients who died within 4 days after admission and those for whom there was no ZIP code.
The patients were divided into two groups: (I) patients who received cyclosporine A and methylprednisolone 500–1,000 mg/day intravenously for 3 days within 4 days after admission (methylprednisolone plus cyclosporine A group); and (II) those who received methylprednisolone 500–1,000 mg/day intravenously for 3 days within 4 days after admission (methylprednisolone alone group).
Baseline characteristics and outcomes
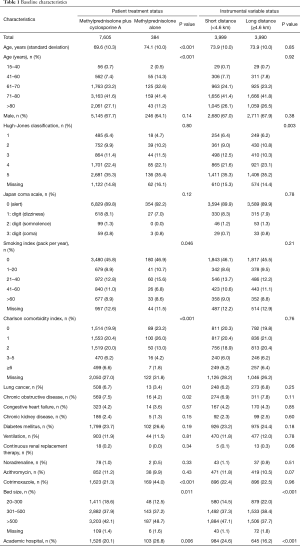
Baseline characteristics included the following: age; sex; Hugh–Jones classification on admission (12); consciousness level on admission; Charlson comorbidity index (CCI); smoking index (packs per year); past history of diabetes mellitus, chronic kidney disease, lung cancer, chronic obstructive pulmonary disease, or congestive heart failure; and use of cotrimoxazole, azithromycin (13), continuous renal replacement therapy, or noradrenaline within 1 day after admission. Patients were categorized into five age groups: 15–40, 41–60, 61–70, 71–80, and >80 years old. Consciousness level on admission was evaluated using the Japan Coma Scale (14,15), which is widely used in Japan, and has been shown to be well correlated with the Glasgow Coma Scale assessment (16). CCI was classified into five groups: 0, 1, 2, 3–5, and ≥6. Smoking index was also categorized into five groups: 0, 1–20, 21–40, 41–60, and >60 pack-years.
The primary outcome was in-hospital mortality. The secondary outcome was length of hospital stay.
Statistical analysis
Continuous variables are presented as mean and standard deviation or median and interquartile range. Categorical variables are presented as number and proportion. In unadjusted comparisons, averages of continuous variables were compared using t-tests, and proportions of categorical variables were compared using χ2 tests.
Some values for the Hugh-Jones classification, CCI, and smoking index were missing, and we therefore performed a multiple imputation procedure to replace each missing value with a set of submitted plausible values, by creating 20 filled-in complete datasets using a Markov chain Monte Carlo algorithm known as chained equations imputation (17). This multiple imputation method assumes that data are missing at random and that any systemic differences between the missing and observed values can be explained by differences in the observed data (18,19).
We performed multivariable logistic regression analyses to evaluate the additional effect of cyclosporine A on the outcomes, adjusting for patient characteristics and hospital characteristics such as bed size and academic hospital. We also performed multivariable logistic regression analyses fitted with generalised estimating equations, adjusting for patient characteristics and for clustering within hospitals.
Linear regression analysis was performed for length of stay, which was natural log-transformed to satisfy the homoscedasticity condition for linear regression. Percent differences and their 95% confidence intervals were estimated by exp (β) – 1, where β denotes the coefficients of the linear regression models.
Instrumental variable analysis
In a properly executed instrumental variable analysis, instrumental variables approximate random assignment of patients to a treatment group analogous to a randomised clinical trial (20,21) (detail in Supplementary 1).
In the present study, ‘differential distance’ was selected as the instrumental variable (22). Differential distance was calculated as the difference between the distance from a patient’s residence to the nearest hospital that administered cyclosporine A to at least one case in the year of treatment, and the distance from a patient’s residence to the nearest hospital of any type. We calculated distances in kilometres between the centres of the two ZIP codes for patient residence. We then created a binary instrumental variable by assigning patients with the median differential distance.
We used a two-stage residual inclusion estimation framework for instrumental variable analysis (23,24). The residual inclusion approach has been shown to generate more consistent and less biased estimates for a variety of nonlinear models.
A P value <0.05 was considered to indicate statistical significance. All statistical analyses were performed using STATA/MP version 14.2 software (STATA Corp., College Station, TX, USA).
Results
We identified 8,375 patients during the study period who received methylprednisolone at a dose of 500–1,000 mg/day for 3 days within 4 days after admission (Figure 1). Among these, 7,989 patients were eligible for this study, including 384 patients who received cyclosporine A and 7,605 patients without cyclosporine A.
Values were missing for smoking index (12.5%), Hugh-Jones classification (14.8%), and CCI (27.2%) (Table 1). Patient background characteristics were significantly different in the methylprednisolone plus cyclosporine A compared with the methylprednisolone alone group with respect to age, smoking index, and CCI. The proportions of patients with lung cancer or chronic obstructive pulmonary disease was significantly lower in the methylprednisolone plus cyclosporine A compared with the methylprednisolone alone group (3.4% vs. 6.7%, P=0.01; 4.2% vs. 7.5%, P=0.02; respectively). Patients in the methylprednisolone plus cyclosporine A group were more likely to receive cotrimoxazole within 1 day after admission than those in the methylprednisolone alone group (44.0% vs. 21.3%, P<0.001).
Full table
The overall in-hospital mortality was 24.9% (1,990/7,989). There was no significant difference between the methylprednisolone plus cyclosporine A group and the methylprednisolone alone group in terms of in-hospital mortality (25.3% vs. 24.9%, P=0.87).
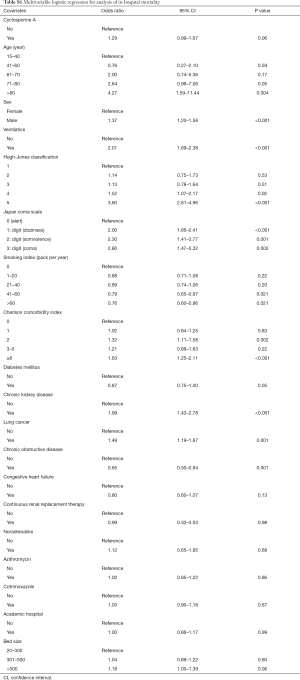
Multivariable logistic regression analysis found no significant difference between the methylprednisolone plus cyclosporine A group and the methylprednisolone alone group with respect to in-hospital mortality [odds ratio, 1.27; 95% confidence interval (CI), 0.99–1.64] (Tables 2 and S1).

Full table
Full table
The median differential distance was 4.6 km. The differential distance was highly associated with the actual receipt of cyclosporine A (F statistic =49.5) but was not significantly associated with in-hospital mortality (coefficient, −0.004; 95% CI, −0.11 to 0.11).
Instrumental variable analysis also found no significant differences between the methylprednisolone plus cyclosporine A group and the methylprednisolone alone group with respect to in-hospital mortality (odds ratio, 0.94; 95% CI, 0.12–7.67) (Table 2). There was no significant difference between the methylprednisolone plus cyclosporine A group and the methylprednisolone alone group with respect to length of stay (percent difference, −43.3%; 95% CI, −81.1 to 70.3) (Table 3).

Full table
Discussion
The present study compared the effectiveness of high-dose methylprednisolone plus cyclosporine A with high-dose methylprednisolone alone for treating patients with AE-IPF, using data from a Japanese national inpatient database. Our analysis showed no significant difference in in-hospital mortality between the two groups.
A previous study found no significant difference in mortality between IPF patients treated with cyclosporine A plus glucocorticoids and cyclophosphamide plus glucocorticoids (25). The population in the present study comprised patients with more severe disease than this previous study. To the best of our knowledge, the present study provides the first evidence regarding the addition of cyclosporine A to glucocorticoids for the treatment of AE-IPF. An advantage of this study included the use of instrumental variable analyses to generate pseudo-randomization adjusting for unmeasured and measured confounders.
The results of the current study showed that the addition of cyclosporine A to systemic glucocorticoids in patients with AE-IPF did not significantly reduce in-hospital mortality compared with systemic glucocorticoids alone. Acute worsening or development of dyspnoea typically of <1-month duration is a diagnostic criterion of AE-IPF. Our study only included inpatients, and the administration of cyclosporine A may thus have been delayed. However, the insignificant difference may reflect a genuine lack of effect of cyclosporine A on AE-IPF.
This study had several limitations. The database did not include data on the patients’ conditions before admission or their physical conditions, laboratory examinations, and imaging test results. However, we used instrumental variable analysis to balance unmeasured confounders between the two treatment groups, and we showed that differential distance was a strong instrumental variable and created a well-balanced distribution of patient backgrounds between the two groups. Second, the diagnosis of IPF was not well validated. However, we selected patients based on components of the revised diagnostic criteria for AE-IPF (1) who received high-dose methylprednisolone.
Conclusions
This instrumental variable analysis using data from a national inpatient database showed that the addition of cyclosporine A to methylprednisolone did not reduce mortality of AE-IPF patients compared with methylprednisolone alone. However, randomised controlled studies are required to confirm the effect of methylprednisolone plus cyclosporine A in patients with AE-IPF.
Supplementary 1: Instrumental variable analysis
In a properly executed instrumental variable analysis, instrumental variables approximate random assignment of patients to a treatment group analogous to a randomised clinical trial.
In the present study, ‘differential distance’ was selected as the instrumental variable. Differential distance was calculated as the difference between the distance from a patient’s residence to the nearest hospital that administered cyclosporine A to at least one case in the year of treatment, and the distance from a patient’s residence to the nearest hospital of any type. The differential distance was the extra distance beyond the closest hospital that a patient would have to travel to arrive at a hospital that administered cyclosporine A at least once in the year of treatment. We calculated distances in kilometres between the centres of the two ZIP codes for patient residence. We then created a binary instrumental variable by assigning patients with the median differential distance.
To assess the validity of differential distance as an instrumental variable, we confirmed that differential distance was highly correlated with the receipt of cyclosporine A (F statistic >10). We also confirmed that differential distance was not associated with the outcome, and examined the covariate balance between the patients assigned low or high differential distances.
We used a two-stage residual inclusion estimation framework for instrumental variable analysis. The residual inclusion approach has been shown to generate more consistent and less biased estimates for a variety of nonlinear models. In the first-stage model, we measured the association between cyclosporine A and differential distance, adjusting for covariates. From this model, we determined the raw residual for each patient by calculating the difference between the model-predicted probability of receiving cyclosporine A and the actual treatment received. The residuals were included as an additional covariate in our second-stage model. In the second-stage model, the association between treatment and the outcome was estimated with adjustment for covariates. All instrumental variable analyses were performed using robust standard errors.
Acknowledgements
H Yasunaga and K Fushimi received grant support from the Japanese government. This work was supported by grants for Research on Policy Planning and Evaluation from the Ministry of Health, Labour and Welfare, Japan; the Ministry of Education, Culture, Sports, Science and Technology, Japan; and the Japan Agency for Medical Research and Development (AMED). The funders had no role in the execution of this study or interpretation of the results.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board of The University of Tokyo [approval number: 3501-(1)]. Because all data were de-identified, the requirement for patient informed consent was waived.
References
- Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis an international working group report. Am J Respir Crit Care Med 2016;194:265-75. [Crossref] [PubMed]
- Atkins CP, Loke YK, Wilson AM. Outcomes in idiopathic pulmonary fibrosis: A meta-analysis from placebo controlled trials. Respir Med 2014;108:376-87. [Crossref] [PubMed]
- Song JW, Hong SB, Lim CM, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 2011;37:356-63. [Crossref] [PubMed]
- Usui Y, Kaga A, Sakai F, et al. A cohort study of mortality predictors in patients with acute exacerbation of chronic fibrosing interstitial pneumonia. BMJ Open 2013;3. [Crossref] [PubMed]
- Huie TJ, Olson AL, Cosgrove GP, et al. A detailed evaluation of acute respiratory decline in patients with fibrotic lung disease: Aetiology and outcomes. Respirology 2010;15:909-17. [Crossref] [PubMed]
- Collard HR, Yow E, Richeldi L, et al. Suspected acute exacerbation of idiopathic pulmonary fibrosis as an outcome measure in clinical trials. Respir Res 2013;14:73. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Wiederrecht G, Lam E, Hung S, et al. The mechanism of action of FK-506 and cyclosporin A. Ann N Y Acad Sci 1993;696:9-19. [Crossref] [PubMed]
- Homma S, Sakamoto S, Kawabata M, et al. Cyclosporin treatment in steroid-resistant and acutely exacerbated interstitial pneumonia. Intern Med 2005;44:1144-50. [Crossref] [PubMed]
- Inase N, Sawada M, Ohtani Y, et al. Cyclosporin A followed by the treatment of acute exacerbation of idiopathic pulmonary fibrosis with corticosteroid. Intern Med 2003;42:565-70. [Crossref] [PubMed]
- Sakamoto S, Homma S, Miyamoto A, et al. Cyclosporin A in the treatment of acute exacerbation of idiopathic pulmonary fibrosis. Intern Med 2010;49:109-15. [Crossref] [PubMed]
- Fletcher CM. The clinical diagnosis of pulmonary emphysema; an experimental study. Proc R Soc Med 1952;45:577-84. [PubMed]
- Oda K, Yatera K, Fujino Y, et al. Efficacy of concurrent treatments in idiopathic pulmonary fibrosis patients with a rapid progression of respiratory failure: an analysis of a national administrative database in Japan. BMC Pulm Med 2016;16:91. [Crossref] [PubMed]
- Ohta T, Waga S, Hajime H, et al. New grading of level of disordered consciousness (author’s translation). No Shinkei Geka 1974;2:623-7. [PubMed]
- Shigematsu K, Nakano H, Watanabe Y. The eye response test alone is sufficient to predict stroke outcome—reintroduction of Japan Coma Scale: a cohort study. BMJ Open 2013;3. [Crossref] [PubMed]
- Ono K, Wada K, Takahara T, et al. Indications for computed tomography in patients with mild head injury. Neurol Med Chir (Tokyo) 2007;47:291-7; discussion 297-8. [Crossref] [PubMed]
- Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med 1991;10:585-98. [Crossref] [PubMed]
- Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [Crossref] [PubMed]
- Li P, Stuart EA, Allison DB. Multiple imputation: a flexible tool for handling missing data. JAMA 2015;314:1966-7. [Crossref] [PubMed]
- Newhouse JP, McClellan M. Econometrics in outcomes research: the use of instrumental variables. Annu Rev Public Health 1998;19:17-34. [Crossref] [PubMed]
- Staiger D, Stock J. Instrumental variables regression with weak instruments. Econometrica 1997;65:557-86. [Crossref]
- Garabedian LF, Chu P, Toh S, et al. Potential bias of instrumental variable analyses for observational comparative effectiveness research. Ann. Intern Med 2014;161:131-8. [Crossref] [PubMed]
- Terza JV, Basu A, Rathouz PJ. Two-stage residual inclusion estimation: Addressing endogeneity in health econometric modeling. J Health Econ 2008;27:531-43. [Crossref] [PubMed]
- Terza JV, Bradford WD, Dismuke CE. The use of linear instrumental variables methods in health services research and health economics: A cautionary note. Health Serv Res 2008;43:1102-20. [Crossref] [PubMed]
- Miyazaki Y, Azuma A, Inase N, et al. Cyclosporine a combined with low-dose corticosteroid treatment in patients with idiopathic pulmonary fibrosis. Respir Investig 2015;53:288-95. [Crossref] [PubMed]