Peri-procedural myocardial infarction is all the same?
Peri-procedural myocardial infarction (MI) and complexed procedures
Peri-procedural MI is one of the important complications after percutaneous coronary intervention (PCI). In general, the incidence of peri-procedural MI was reported as 2% to 30%, which depends on the definitions of peri-procedural MI (1). These definitions use the different types of cardiac marker measured, the different thresholds of these markers for diagnosis, and additional clinical criteria. Although the pathophysiology of peri-procedural MI are not fully understood, distal embolization of thrombus or plaques, and occlusion of small side branch has been well known to contribute to peri-procedural MI (1). The most relevant risk factors are the complexed lesions (i.e., calcified lesion, high SYNTAX score, and large necrotic core), and complex procedures (i.e., multiple lesions and usage of rotational atherectomy) (1). Previous studies showed that other characteristics such as old age, diabetes mellitus (DM), renal dysfunction and left ventricular dysfunction are also associated with peri-procedural MI (1). Additionally, those risk factors interact with each other and contribute to peri-procedural MI. Patients who require rotational atherectomy during the procedure usually have multiple risk factors of peri-procedural MI (2). These patients are more likely to be high age, and have the atherosclerotic risk factors (DM, hypertension and hyperlipidemia) and renal dysfunction (2). The lesions that require rotational atherectomy are frequently associated with calcified plaques, long lesions and multiple lesions (2). Moreover, rotational atherectomy is associated with the higher incidence of slow flow phenomenon (3). The mechanism of this phenomenon has been described as platelet aggregation, micro-vessel obstruction, release of vasoactive substances, atheromatous debris and vasospasm. Slow flow phenomenon results in myocardial hypoperfusion, and secondary to peri-procedural MI (4). Therefore, peri-procedural MI frequently occurred in the patients with rotational atherectomy (4). The decision of how the patients with peri-procedural MI should be treated (i.e., longer hospitalization with monitor, additional medical treatment, and coronary angiography) must be made soon. Therefore, the important first-step is the awareness of peri-procedural MI after PCI so that the patients with peri-procedural MI are properly and promptly treaded after procedure without any delay.
Different sensitivity of peri-procedural MI based on definitions
In the last decades, several definitions have been introduced to identify peri-procedural MI. The World Health Organization (WHO) defined MI based on symptoms, electrocardiograph (ECG) abnormality and elevation of creatine kinase (CK) (5). As technologies have been developed, more sensitive and specific myocardial cardiac marker became available, allowing the detection of small amount of myocardial injury or necrosis. In 2000, the European and American cardiology societies proposed the first universal definition based on the elevation of troponin levels, which noted that any necrosis related to myocardial ischemia is recognized as MI (6). In 2007, the second definition was established to raise the thresholds for troponin levels, implying the different causes resulting in MI (7). Moreover, with the development of more sensitive cardiac marker for myocardial necrosis and advanced imaging devices, in 2012, the current third definition was introduced. This definition not only raised the thresholds more but also required clinical symptoms, ECG abnormality, or angiographic flow-limiting findings or imaging evidence of additional loss of viable myocardium (5). Additionally, this current universal definition defined myocardial injury as ischemia associated to the isolated elevation of troponin levels without ischemic findings, or cardiovascular events (5). Recently, the Society for Cardiovascular Angiography and Intervention (SCAI) proposed peri-procedural MI criteria similar to MI criteria in the setting of post-coronary artery bypass graft, which require more than 10 times upper reference limit (URL) for CKMB and/or more than 70 times URL for troponin (8). Table 1 summarized transition of each definition. Additionally, the differences among these definitions result in variation in detection of peri-procedural MI (18–44% in the second universal definition, 2–20% in the third universal definition and 2–6% in the SCAI definition) (9-13). Definition of peri-procedural MI still remains controversial in the high sensitive troponin era.
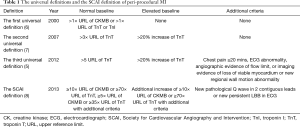
Full table
Association between peri-procedural MI and prognosis
In addition to the controversy of its definition, the clinical relevance of peri-procedural MI has also been an issue. Previous several studies showed association between peri-procedural MI and increased clinical adverse events, while the others failed to show it. In the sub-study from TRITON-TIMI 38 where peri-procedural MI was defined as CK-MB levels above three times the URL on two samples within 48 h of PCI, or above five times the URL on a single sample, the relationship between peri-procedural MI and cardiovascular death at 6 months was investigated. Among 13,608 acute coronary syndrome (ACS) patients, 600 patients with peri-procedural MI (3.2%) were associated with higher cardiovascular death relative to those without peri-procedural MI [hazard ratio (HR): 2.1, 95% confidence interval (CI): 1.3–4.4, P=0.004] (14). Furthermore, the BARI 2D trial enrolling total 2,368 patients with PCI or CABG where peri-procedural MI was defined as increase in CK-MB levels above three times the URL after PCI and a tenfold increase after CABG, showed peri-procedural MI observed in 2.2% was the higher risk of cardiac death as compared to patients without any MI (HR, 3.4; P<0.008) (15). On the other hand, in the ACUITY trial enrolling 7,773 patients with non-ST elevated MI, 466 patients (6.0%) who experienced peri-procedural MI, which was defined as new increase of CK-MB levels above three times the URL, did not show the higher risk of cardiac death than non-MI patients (adjusted HR, 1.3; 95% CI: 0.85–1.98, P=0.22) (16). Moreover, a sub-study of the EVENT registry enrolling 4,623 stable angina patients evaluated the incidence of peri-procedural MI and prognosis in those patients (17). Peri-procedural MI was defined as an elevation above three times the URL in the peak values of CK-MB and troponin I or T after PCI. Peri-procedural MI occurred in 357 (7.7%) based on the CK-MB criteria and 1,198 (25.9%) based on the troponin criteria. Peri-procedural MI according to the CK-MB criteria and troponin as continuous values was associated with the increase of mortality (adjusted HR, 1.4, 95% CI: 1.2–1.6 and adjusted HR 1.4, 95% CI: 1.2–1.5, respectively). However, increase above three times the URL of CK-MB was more strongly associated with mortality than that of troponin (adjusted HR, 2.5, 95% CI: 1.5–4.1 and adjusted HR, 1.7, 95% CI: 1.1–2.5, respectively). Those studies varied from stable angina to NSTEMI, selection of CK or troponin in individual definition and threshold of CK, CK-MB, or troponin, which may contribute to different effects of peri-procedural MI on clinical outcomes. Even when using the third definition, there are still similar controversial relationship between peri-procedural MI and outcomes (9-13,18,19) (Table 2). Baker et al. evaluated the accuracy of the 2 definitions (second and third definitions) (10). Of 7,333 patients, peri-procedural MI in third and second definitions occurred in 154 (2.1%) and 2,339 patients (31.9%), respectively. Both definitions were not associated with predictors of peri-procedural MI [odd ratio (OR), 1.4; 95% CI, 0.7–3.0; P=0.38 and OR, 1.1; 95% CI: 0.9–1.5; P=0.34, respectively]. The receiver-operating characteristic (ROC) curves show only a modest correlation for both definitions to predict death or MI without any improvement for the 2012 definition (ROC curve in both definitions: 0.72). However, in 2018, more recent prospective observational trial enrolling 1390 elective PCI patients evaluated the association between peri-procedural MI defined as the third universal definition and myocardial injury (11). Peri-procedural MI and myocardial injury occurred in 7% and 22%, respectively. The patients with peri-procedural MI or myocardial injury had high rate of ischemic events composed of cardiovascular death, MI, ischemia stroke, and refractory angina (adjusted HR, 1.7, 95% CI: 1.1–2.6; P=0.004). However, in this study, the impact of myocardial injury on clinical outcomes and comparison of clinical outcomes between myocardial injury and peri-procedural MI were not investigated. Therefore, this did not directly indicate association between peri-procedural MI and outcomes.
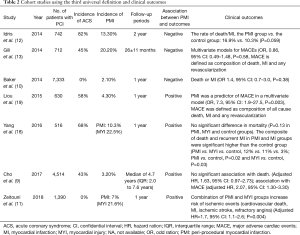
Full table
What we learned from this study?
This study represents the first single, prospective study enrolling 58 near-consecutive patients with stable coronary artery disease treated with rotational atherectomy for calcified plaque lesion, and investigated the incidence of peri-procedural MI in the third universal definition by using cardiac magnetic resonance imaging (CMR) (20). CMR was performed at 7 days after the procedure and 6 months as follow-up. First, McEntegart et al. showed difference of sensitivity between SCAI definition and the third universal definition of peri-procedural MI in patients requiring rotational atherectomy, which was consistent with previous studies. Second, even if using the third universal definition, there was a distinct difference in detection of peri-procedural MI between criteria with and without CMR (24% vs. 10%, respectively) (20). CMR presumably reclassify the myocardial injury patients without CMR into the peri-procedural MI patients. Third, myocardial edema and wall motion abnormality observed at 7 days (51% and 22%, respectively) disappeared at the follow-up of 6 months (0% and 0%), while late gadolinium enhance (LGE) at 7 days (16%) was mostly observed at 6 months (14%). This suggests the differences between transient myocardial injury and persisted MI in CMR. In addition, there is a concern that the excessive inclusion of peri-procedural MI might execute in combining good and poor prognostic patients into one category, although the influence of the differences on outcomes is also unclear.
Contribution of CMR in third universal definition
The presence of ECG changes or symptoms is not so highly sensitive to diagnose MI. Indeed, ECG in the patients with NSTEMI does not always show abnormality. Additionally, silent MIs are common, compromising as many as 7–30% in all MI (8). Chest pain after PCI frequently happens in the absence of elevated cardiac marker, which has been thought to be non-ischemia in origin. Therefore, the absence of ECG abnormality and ischemic symptom is not reliable to diagnose and exclude peri-procedural MI. Regarding wall motion abnormality, it may not occur unless the infarcted region exceeds 20–50% of the myocardial wall (21). Moreover, peri-procedural MI region is generally small. Porto et al. reported that the incidence of peri-procedural MI was 23% following PCI, but the mean infarcted size in those MI was only 5% of the left ventricular mass (22). Therefore, an area of peri-procedural MI is not large enough for the wall motion abnormality to be recognized.
In the midst of development of technology in magnetic resonance image field, the data has been accumulated and shown a well validated technique for detection and assessment of MI. Several previous studies using animal models showed a nearly exact correlation of size and shape of infarcted myocardium between delayed enhancement CMR and histopathology (23). These studies also reported that delayed enhancement CMR is able to distinguish between reversible and irreversible injury independent of wall motion, infarct age, and reperfusion status. Previous human studies showed relationship between infarcted region size by delayed enhancement CMR and peak CK-MB, troponin I and measurements by positron emission tomography (24-26). CMR is similar to single-photon emission computed tomography (SPECT) in terms of detection transmural MI, but superior to SPECT in subendocardial infarction (27). These data support the higher incidence of peri-procedural MI assessed with CMR in third universal definition than non-CMR in the report by McEntegart et al.
Further task
The data presented by McEntegart et al. are valuable, because they have taught us that the different levels of sensitivity existing in the same definition depend on additional criteria, except for the troponin criteria. However, this data does not support routine usage of CMR when diagnosing peri-procedural MI. As mentioned above, the evidence for prognostic implications of peri-procedural MI based on large studies are inconsistent. Therefore, the definition and effects of peri-procedural MI on clinical outcomes still remain controversial. Large peri-procedural MI appears to be associated with worse clinical outcomes, which should be similar to spontaneous MI. However, there are no established cutoff values for cardiac troponin and infarcted size of peri-procedural MI which can have prognostic relevance. As this study showed, it still remains unclear whether the effect of transient wall motion abnormality in CMR on prognostic relevance is different from that of the positive LGE findings. Are there any differences of risks in patients filling current peri-procedural MI criteria in terms of effects on clinical outcomes? Prognostic risks might need to be stratified in the patients filling criteria of peri-procedural MI in the third universal definition. Further studies are needed to investigate these unsolved issues.
Acknowledgements
The authors thank Kay Paek in CV Path Institute for assistance with this work.
Footnote
Conflicts of Interest: Dr. Jinnouchi has received speaking honoraria from Boston Scientific. Dr. Sakakura has received speaking honoraria from Abbott Vascular, Boston Scientific, Medtronic Cardiovascular, Terumo, OrbusNeich, and NIPRO; has served as a proctor for Rotablator for Boston Scientific; and has served as a consultant for Abbott Vascular and Boston Scientific. Another author has no conflicts of interest to declare.
References
- Herrmann J. Peri-procedural myocardial injury: 2005 update. Eur Heart J 2005;26:2493-519. [Crossref] [PubMed]
- Jinnouchi H, Kuramitsu S, Shinozaki T, et al. Five-Year Clinical Outcomes After Drug-Eluting Stent Implantation Following Rotational Atherectomy for Heavily Calcified Lesions. Circ J 2018;82:983-91. [Crossref] [PubMed]
- Sakakura K, Funayama H, Taniguchi Y, et al. The incidence of slow flow after rotational atherectomy of calcified coronary arteries: A randomized study of low speed versus high speed. Catheter Cardiovasc Interv 2017;89:832-40. [Crossref] [PubMed]
- Tomey MI, Kini AS, Sharma SK. Current status of rotational atherectomy. JACC Cardiovasc Interv 2014;7:345-53. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581-98. [Crossref] [PubMed]
- Alpert JS, Thygesen K, Antman E, et al. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000;36:959-69. [Crossref] [PubMed]
- Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol 2007;50:2173-95. [Crossref] [PubMed]
- Moussa ID, Klein LW, Shah B, et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol 2013;62:1563-70. [Crossref] [PubMed]
- Cho MS, Ahn JM, Lee CH, et al. Differential Rates and Clinical Significance of Periprocedural Myocardial Infarction After Stenting or Bypass Surgery for Multivessel Coronary Disease According to Various Definitions. JACC Cardiovasc Interv 2017;10:1498-507. [Crossref] [PubMed]
- Baker NC, Lipinski MJ, Escarcega RO, et al. Definitions of periprocedural myocardial infarction as surrogates for catheterization laboratory quality or clinical trial end points. Am J Cardiol 2014;113:1326-30. [Crossref] [PubMed]
- Zeitouni M, Silvain J, Guedeney P, et al. Periprocedural myocardial infarction and injury in elective coronary stenting. Eur Heart J 2018;39:1100-9. [Crossref] [PubMed]
- Idris H, Lo S, Shugman IM, et al. Varying definitions for periprocedural myocardial infarction alter event rates and prognostic implications. J Am Heart Assoc 2014;3. [Crossref] [PubMed]
- Gili S, D'Ascenzo F, Moretti C, et al. Impact on prognosis of periprocedural myocardial infarction after percutaneous coronary intervention. J Interv Cardiol 2014;27:482-90. [Crossref] [PubMed]
- Bonaca MP, Wiviott SD, Braunwald E, et al. American College of Cardiology/American Heart Association/European Society of Cardiology/World Heart Federation universal definition of myocardial infarction classification system and the risk of cardiovascular death: observations from the TRITON-TIMI 38 trial (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis in Myocardial Infarction 38). Circulation 2012;125:577-83. [Crossref] [PubMed]
- Chaitman BR, Hardison RM, Adler D, et al. The Bypass Angioplasty Revascularization Investigation 2 Diabetes randomized trial of different treatment strategies in type 2 diabetes mellitus with stable ischemic heart disease: impact of treatment strategy on cardiac mortality and myocardial infarction. Circulation 2009;120:2529-40. [Crossref] [PubMed]
- Prasad A, Gersh BJ, Bertrand ME, et al. Prognostic significance of periprocedural versus spontaneously occurring myocardial infarction after percutaneous coronary intervention in patients with acute coronary syndromes: an analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J Am Coll Cardiol 2009;54:477-86. [Crossref] [PubMed]
- Novack V, Pencina M, Cohen DJ, et al. Troponin criteria for myocardial infarction after percutaneous coronary intervention. Arch Intern Med 2012;172:502-8. [Crossref] [PubMed]
- Yang X, Tamez H, Lai C, et al. Type 4a myocardial infarction: Incidence, risk factors, and long-term outcomes. Catheter Cardiovasc Interv 2017;89:849-56. [Crossref] [PubMed]
- Liou K, Jepson N, Kellar P, et al. Prognostic Significance of Peri-procedural Myocardial Infarction in the Era of High Sensitivity Troponin: A Validation of the Joint ACCF/AHA/ESC/WHF Universal Definition of Type 4a Myocardial Infarction with High Sensitivity Troponin T. Heart Lung Circ 2015;24:673-81. [Crossref] [PubMed]
- McEntegart M, Corcoran D, Carrick D, et al. Incidence of Procedural Myocardial Infarction and Cardiac Magnetic Resonance imaging-detected myocardial injury follwing Percutaneous Coronary Intervention with Rotational Atherectomy. EuroIntervention 2018. [Epub ahead of print]. [PubMed]
- Mahrholdt H, Wagner A, Parker M, et al. Relationship of contractile function to transmural extent of infarction in patients with chronic coronary artery disease. J Am Coll Cardiol 2003;42:505-12. [Crossref] [PubMed]
- Porto I, Selvanayagam JB, Van Gaal WJ, et al. Plaque volume and occurrence and location of periprocedural myocardial necrosis after percutaneous coronary intervention: insights from delayed-enhancement magnetic resonance imaging, thrombolysis in myocardial infarction myocardial perfusion grade analysis, and intravascular ultrasound. Circulation 2006;114:662-9. [Crossref] [PubMed]
- Kim RJ, Fieno DS, Parrish TB, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100:1992-2002. [Crossref] [PubMed]
- Ricciardi MJ, Wu E, Davidson CJ, et al. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation 2001;103:2780-3. [Crossref] [PubMed]
- Selvanayagam JB, Porto I, Channon K, et al. Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury: insights from cardiovascular magnetic resonance imaging. Circulation 2005;111:1027-32. [Crossref] [PubMed]
- Klein C, Nekolla SG, Bengel FM, et al. Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: comparison with positron emission tomography. Circulation 2002;105:162-7. [Crossref] [PubMed]
- Wagner A, Mahrholdt H, Holly TA, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet 2003;361:374-9. [Crossref] [PubMed]


