Myocardial performance index for detection of subclinical abnormalities in patients with sarcoidosis
Introduction
Sarcoidosis is a granulomatous disease of unknown cause that involves multiple organ systems, including the lungs, heart, skin, and eyes (1). Involvement of the heart in sarcoidosis may lead to diastolic dysfunction, left ventricular wall motion abnormalities, reduced systolic function, cardiac arrhythmias, and sudden death (1-4).
Although sarcoidosis affects many organs, the most prominent complications are ventricular diastolic and systolic dysfunction and accounts for most of the morbidity, mortality, and sudden death (5). The treatment approach of sarcoidosis should aim at relieving symptoms, controlling the inflammatory process, preventing further worsening in myocardial function and preventing sudden death. Physicians should not wait until the onset of symptoms to start therapy; the treatment should be started as soon as the diagnosis is made (5). Therefore, early recognition of cardiac abnormalities in these patients is very important for modifying their treatment and improving morbidity and mortality.
‘To date, there are some reports investigating the abnormalities of the cardiac functions in patients with sarcoidosis in the literature (6-8). Conventional echocardiographic methods have been used for the assessment of cardiac function in these trials. However, several controversial results were obtained. Hence, a new method for more objectively estimating the cardiac functions in sarcoidosis is needed.’
Current developments in cardiac ultrasound permitted more precise echocardiographic assessment of cardiac functions. Tissue Doppler imaging (TDI) is a recently developed technique for the quantization of myocardial velocities in the left and right ventricle using low-velocity pulsed wave Doppler interrogation of the myocardium. Recently, a new non-invasive Doppler-derived myocardial performance index (MPI) was proposed by Tei et al. (9,10). MPI, which combines both systolic and diastolic function, may give a better reflection of the global left ventricular function than an isolated evaluation of either ejection or relaxation. Increased MPI was shown to be a prognostic index and independent predictor for cardiac death in various heart diseases (11-13).
The aim of this study was to evaluate ventricular functions in patients with sarcoidosis without an obvious heart disease by using tissue Doppler-derived left and right ventricular MPI and to validate this index against conventional measures of systolic and diastolic left ventricular function and pulmonary artery pressure.
Materials and methods
Study population
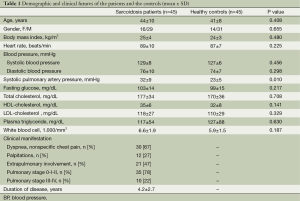
The study population included 45 consecutive patients with sarcoidosis (29 men; mean age, 44±10 years, mean disease duration, 4.2±2.7 years) and 45 healthy subjects as controls (31 men; mean age, 41±8 years). The patients were referred from our Chest Disease Department. The diagnosis of sarcoidosis was established according to the American Thoracic Society (ATS) recommendations (14). Age, gender, and body mass index (BMI) were recorded. Fasting blood glucose, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglyceride levels, and white blood cell count were recorded. The demographic characteristics, clinical features, and MRI findings of the patients and the controls are given in Table 1.
Full table
The control subjects had no cardiovascular or any other organ system disease and had normal physical examination, chest roentgenogram, electrocardiogram, and two-dimensional and Doppler echocardiogram. None of the patients had hypertension, renal failure, diabetes mellitus, left ventricle (LV) ejection fraction (EF) lower than 50%, severe valvular regurgitation and moderate or severe valvular stenosis, coronary artery disease, chronic obstructive pulmonary disease, and atrial fibrillation. The patients with poor echocardiographic window were also excluded. This study complied with the Declaration of Helsinki, was approved by the Ethics Committee and the institutional review board of Erciyes University Medical School, and informed consent was obtained from each patient.
Echocardiography
All patients underwent complete transthoracic echocardiographic studies including two-dimensional, color flow, and spectral Doppler as well as TDI with a GE-Vingmed Vivid 7 system (GE-Vingmed Ultrasound AS, Horten, Norway) using a 2.5-MHz transducer. Echocardiographic measurements were taken with patients in the left lateral decubitus position using standard parasternal long- and short-axis and apical views. At least three consecutive beats in sinus rhythm were recorded, and the average values were taken. All measurements were taken according to the American Society of Echocardiographists’ recommendations (15). The LV end-diastolic and end-systolic dimensions (LVEDD and LVESD), interventricular septal and posterior wall thickness (IVSd and LPWd) were measured from M-mode recordings of LV cavity with the cursor at the tip of the mitral valve leaflets in the parasternal long axis view. Left ventricular EF was calculated using a modification of Simpson’s rule. RV end-diastolic dimension, right and left atrial dimensions were measured in the apical four chamber view. The LV mass was calculated with Devereux formula. Pulmonary arterial systolic pressure (PASP) was calculated by the following formulas (16): RVSP =4 × (peak tricuspid regurgitation velocity)2 + RAP. PASP = RVSP - peak PV gradient where RVSP = right ventricular systolic pressure, and RAP = right atrial pressure (assessed by inferior vena cava size and collapsibility). Pulmonary hypertension (PH) was diagnosed if systolic pulmonary artery pressure exceeded the upper limits of normal for age- and BMI-adjusted reference ranges (17).
A 2 mm sized sample volume of pulsed wave Doppler was placed at the tip of mitral and tricuspid leaflets to record trans-mitral and trans-tricuspid Doppler velocities. Peak early diastolic velocity (E), peak atrial filling velocity (A), E/A ratio, E wave deceleration time (DT) and isovolumic relaxation time (IVRT) were measured from the LV and RV filling recordings.
Myocardial tissue Doppler velocities [peak systolic (Sa), early diastolic (Ea), and late diastolic velocities (Aa)] were recorded using spectral pulsed Doppler from the LV free wall, septum, and RV free wall from the apical four chamber view (18). The left and right ventricular MPI was calculated as the sum of the isometric contraction time and isometric relaxation time divided by ventricular ejection time (9,10). All echocardiographic measurements were carried out by two experienced observers who were unaware of the clinical data.
Reproducibility of the measurements
Intraobserver and intraobserver variability were assessed in 12 randomly chosen patients. Variability was calculated as the mean percent error, derived as the difference between two sets of measurements, divided by the mean of the observations. Both investigators were blinded to the patients’ diagnosis.
Statistical analysis
Continuous variables were given as mean ± SD; categorical variables were defined as percentage. Independent-sample t test was used to compare the study variables between sarcoidosis patients and control subjects. Correlation analyses were performed using the Pearson coefficient of correlation. Receiver-operating characteristic (ROC) curve was used to determine best cutoff value of left ventricular MPI for prediction of left ventricular diastolic dysfunction. A probability value of P<0.05 was considered significant, and two-tailed P values were used for all statistics. All statistical analyses were carried out using statistical software (SPSS, version 13.0 for Windows; SPSS, Chicago, IL, USA).
Results
Clinical features
According to the basic clinical and demographic characteristics, both groups of the study were similar with regard to age, BMI, fasting glucose, white blood cell count, cholesterol level, and smoking status. All subjects were normotensive and no significant differences in systolic or diastolic blood pressures and heart rate between these two groups were observed. However, systolic pulmonary arterial pressure was significantly higher in sarcoidosis patients than in healthy controls (32±9 vs. 23±5 mmHg, P=0.010) (Table 1). Patients had received diagnoses of sarcoidosis a mean time of 4.2±2.7 years prior to study enrollment, and 94% of patients had pulmonary involvement.
Echocardiographic data
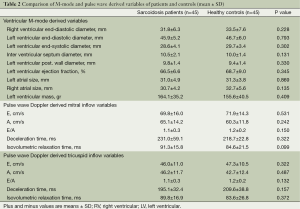
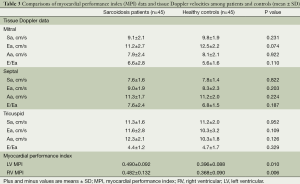
Comparisons of the baseline echocardiographic values among sarcoidosis patients and healthy controls are shown in Tables 2 and 3.
Full table
Full table
2D echocardiography and standard doppler flow measurements
The results of conventional echocardiographic examinations are summarized in Table 2. The LV diameters, EF, and LV diastolic filling parameters such as A wave, DT, and isovolumetric relaxation time except E wave and E/A ratio were comparable in sarcoidosis patients and healthy controls. RV diameters and tricuspid diastolic velocities (E and A), DT, and isovolumetric relaxation time except E wave and E/A ratio were also similar in both groups.
Tissue doppler and MPI data
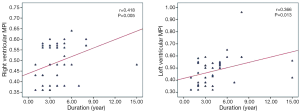
Comparisons of the tissue Doppler parameters and MPI values among sarcoidosis patients and healthy controls are also summarized in Table 3. Systolic velocities of the both ventricles (Sa) were not different between the groups. Similarly, Ea, Aa, and E/Ea values were also comparable between the groups. “Left ventricular MPI (0.490±0.092 vs. 0.396±0.088, P=0.010) and right ventricular MPI (0.482±0.132 vs. 0.368±0.090, P=0.006) were significantly higher in patients with sarcoidosis than the control subjects”. There was a correlation between the disease duration and left and right ventricular MPI (r=0.418, P=0.005; r=0.366, P=0.013, respectively) (Figure 1). There was also a correlation between the systolic pulmonary arterial pressure and right ventricular MPI but not left ventricular MPI (r=0.370, P=0.012; r=0.248, P=0.109, respectively) (Figure 2). Left ventricular diastolic dysfunction was detected in 18 (40%) of 45 patients; 13 patients presented with diastolic dysfunction I and 5 patients presented with diastolic dysfunction II.

On the basis of the ROC analysis, the cutoff value of left ventricular MPI >0.46 had 92% sensitivity and 64% specificity in predicting left ventricular diastolic dysfunction (Figure 3).

Intraobserver and interobserver variability
Intraobserver and interobserver variability for conventional Doppler and TDI-derived variables (LVEDD, LPWD, Sm, Em, Am, and MPI) ranged from 3% to 6%.
Discussion
In this study, we investigated the ventricular functions in patients with sarcoidosis by using high-usefulness TDI. We have demonstrated that tissue Doppler-derived left and right ventricular MPI were impaired in sarcoidosis patients, although systolic and diastolic function parameters were comparable in the patients and controls. We also showed a correlation between the systolic pulmonary arterial pressure and right ventricular MPI in patients with sarcoidosis. According to this study, left ventricular MPI >0.46 measured predicts left ventricular diastolic dysfunction with 92% sensitivity and 64% specificity.
There are only a few reports in the literature related to LV diastolic function in patients with sarcoidosis (6-8). The first related reports determined diastolic dysfunction according to the criteria based on the amount of patients who had E/A <1 in conventional Doppler echocardiography. Fahy et al. (6) studied 50 patients with sarcoidosis. Seven patients (14%) had diastolic dysfunction by mitral inflow pattern. In another study, Ucar et al. (7) examined 30 patients with pulmonary sarcoidosis in their prospective study. They found left ventricular diastolic dysfunction in 16 patients (63.3%) by pulse wave Doppler echocardiography. Sköld et al. (8) showed the presence of both systolic and diastolic dysfunction in patients with cardiac sarcoidosis documented by Doppler echocardiography and magnetic resonance imaging. Diastolic dysfunction was found in 56-59% of the patients. In our study, left ventricular diastolic dysfunction was detected in 18 (40%) of 45 patients by mitral inflow pattern.
Several mechanisms may be responsible for ventricular dysfunction in patients with sarcoidosis. One of the main possible mechanisms is cardiac infiltration by sarcoid granulomas which may cause left ventricular diastolic dysfunction due to ventricular stiffness or reduced systolic contractile function, or both (5).
MPI, as an echocardiographic parameter summarizing right and left ventricular systolic and diastolic function, was measured as the sum of the isometric contraction time and isometric relaxation time divided by ventricular ejection time (9). In this study, to find out more accurate and objective evaluation of cardiac changes in patients with sarcoidosis, we have used tissue Doppler-derived MPI in addition to conventional echocardiographic measurements and other tissue Doppler modalities. We used MPI for the reason that MPI is easily measurable, reproducible, and have been clinically helpful in assessing global ventricular function in both left and right ventricles. The MPI is independent of heart rate and ventricular geometry and also superior to conventional methods, because it is relatively unaffected by significant changes in preload and afterload (9,10). In addition, MPI might be more accurate and reflective of overall cardiac dysfunction than systolic and diastolic measures alone. As a result, this new modality might be more sensitive for detecting subclinical abnormalities (19,20).
To our knowledge, there is only one report in the literature of ventricular function evaluated by MPI in patients with sarcoidosis (21). However, several studies have evaluated ventricular function using MPI in patients with PH, Behcet’s disease, mitral stenosis, hypertension, and diabetes mellitus (22-26). Dyer et al. (22) reported that patients with idiopathic pulmonary artery hypertension had worse RV and LV MPI than healthy volunteers. Tavil et al. (23) also showed that RV MPI patients with Behcet’ disease had higher RV and LV MPI values. Ozdemir et al. (24) demonstrated that TDI-derived RV MPI correlates well with pulmonary arterial pressure in patients with mitral stenosis. Karvounis and Yilmaz et al. showed that patients with hypertension and diabetes mellitus had higher ventricular MPI compared with healthy volunteers (25,26). Our study demonstrates that ventricular MPI of both ventricles were higher in patients with sarcoidosis than in healthy controls.
In our study, we also showed preserved RV systolic function in all sarcoidosis patients, as indicated by normal tricuspid annular systolic myocardial velocities. However, RV diastolic function was impaired in sarcoidosis patients, as indicated by increased late tricuspid annular velocity and a reduced ratio of early to late tricuspid annular velocities. We confirmed this RV diastolic dysfunction with assessment of RV MPI. While RV systolic function was found to be preserved in patients with sarcoidosis, the increased RV MPI is most likely explained by decreased diastolic function. The diastolic dysfunction of the right ventricle in patients with sarcoidosis seems to be an early subclinical manifestation of myocardial diastolic derangement, similar to that previously reported in other cardiac disease, including systemic hypertension and hypertrophic cardiomyopathy (27,28).
In the current study, we evaluated the left ventricular function using TDI and we calculated TDI-derived MPI in sarcoidosis patients. We demonstrated that LV systolic and diastolic functions are preserved in sarcoidosis patients, as indicated by normal mitral annular systolic and diastolic myocardial velocities and normal ratio of early to late mitral annular diastolic velocities.
PH is a well-known complication of sarcoidosis. The prevalence of PH has been reported to range from 4% to 44% in patients with sarcoidosis (29,30). Handa et al. (30) found that the frequency of PH was 5.7% evaluated with Doppler echocardiography in sarcoidosis patients. In our study, ten patients (22%) have PH and our results were comparable with previous studies. We found also a correlation between the systolic pulmonary arterial pressure and right ventricular MPI. Consequently, RV MPI can be considered noninvasive parameter in monitoring the progression of the disease.
The diastolic dysfunction of right ventricle may be a result of increased RV afterload due to PH. Pulmonary parenchymal involvement in sarcoidosis causes fibrosis and devastation of the pulmonary vessels, resulting in an irreversibly obliterated pulmonary vascular bed. Meanwhile, vascular involvement of sarcoidosis and extrinsic compression of pulmonary arteries by enlarged mediastinal lymph nodes can cause PH in the absence of significant pulmonary fibrosis can also cause PH in Sarcoidosis.
In addition, we also found a weak but significant association between disease duration and MPI in patients with sarcoidosis. If the patients had more disease duration, this correlation would be more prominent. For that reason, further studies that examine the long-term outcomes of cardiac involvements are needed to elucidate this association.
Conclusions
MPI is useful index to evaluate right and left ventricular functions in patients with sarcoidosis. In the present study, increased MPI in both ventricles, whereas LV EF was normal, showed a subclinic impaired ventricular functions in patients with sarcoidosis.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Valantine H, McKenna WJ, Nihoyannopoulos P, et al. Sarcoidosis: a pattern of clinical and morphological presentation. Br Heart J 1987;57:256-63. [PubMed]
- Burstow DJ, Tajik AJ, Bailey KR, et al. Two-dimensional echocardiographic findings in systemic sarcoidosis. Am J Cardiol 1989;63:478-82. [PubMed]
- Reuhl J, Schneider M, Sievert H, et al. Myocardial sarcoidosis as a rare cause of sudden cardiac death. Forensic Sci Int 1997;89:145-53. [PubMed]
- Mehta D, Lubitz SA, Frankel Z, et al. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest 2008;133:1426-35. [PubMed]
- Bargout R, Kelly RF. Sarcoid heart disease: clinical course and treatment. Int J Cardiol 2004;97:173-82. [PubMed]
- Fahy GJ, Marwick T, McCreery CJ, et al. Doppler echocardiographic detection of left ventricular diastolic dysfunction in patients with pulmonary sarcoidosis. Chest 1996;109:62-6. [PubMed]
- Ucar ZZ, Erbaycu A, Pinar A, et al. Significant prevalence of left ventricular diastolic dysfunction in patients with sarcoidosis. Turkish Respiratory Journal 2008;9:4-9.
- Sköld CM, Larsen FF, Rasmussen E, et al. Determination of cardiac involvement in sarcoidosis by magnetic resonance imaging and Doppler echocardiography. J Intern Med 2002;252:465-71. [PubMed]
- Tei C, Ling LH, Hodge DO, et al. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function--a study in normals and dilated cardiomyopathy. J Cardiol 1995;26:357-66. [PubMed]
- Tei C, Nishimura RA, Seward JB, et al. Noninvasive Doppler-derived myocardial performance index: correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr 1997;10:169-78. [PubMed]
- Ascione L, De Michele M, Accadia M, et al. Myocardial global performance index as a predictor of in-hospital cardiac events in patients with first myocardial infarction. J Am Soc Echocardiogr 2003;16:1019-23. [PubMed]
- Dujardin KS, Tei C, Yeo TC, et al. Prognostic value of a Doppler index combining systolic and diastolic performance in idiopathic-dilated cardiomyopathy. Am J Cardiol 1998;82:1071-6. [PubMed]
- Koç F, Tokaç M, Kaya C, et al. Diastolic functions and myocardial performance index in obese patients with or without metabolic syndrome: a tissue Doppler study. Turk Kardiyol Dern Ars 2010;38:400-4. [PubMed]
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999;160:736-55. [PubMed]
- Quiñones MA, Otto CM, Stoddard M, et al. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr 2002;15:167-84. [PubMed]
- Oh JK, Sewald SB, Tajik AJ. eds. Pulmonary hypertension. In: The Echo Manual. 2nd edition. Philadelphia, PA: Lippincott-Raven, 1999:215-22.
- McQuillan BM, Picard MH, Leavitt M, et al. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation 2001;104:2797-802. [PubMed]
- Chen QM, Li W, O’Sullivan C, et al. Clinical in vivo calibration of pulse wave tissue Doppler velocities in the assessment of ventricular wall motion. A comparison study with M-mode echocardiography. Int J Cardiol 2004;97:289-95. [PubMed]
- Choong CY, Herrmann HC, Weyman AE, et al. Preload dependence of Doppler-derived indexes of left ventricular diastolic function in humans. J Am Coll Cardiol 1987;10:800-8. [PubMed]
- Oki T, Tabata T, Yamada H, et al. Clinical application of pulsed Doppler tissue imaging for assessing abnormal left ventricular relaxation. Am J Cardiol 1997;79:921-8. [PubMed]
- Moyssakis I, Gialafos E, Tentolouris N, et al. Impaired aortic elastic properties in patients with systemic sarcoidosis. Eur J Clin Invest 2008;38:82-9. [PubMed]
- Dyer KL, Pauliks LB, Das B, et al. Use of myocardial performance index in pediatric patients with idiopathic pulmonary arterial hypertension. J Am Soc Echocardiogr 2006;19:21-7. [PubMed]
- Tavil Y, Ozturk MA, Sen N, et al. The assessment of cardiac functions by tissue Doppler-derived myocardial performance index in patients with Behcet’s disease. Clin Rheumatol 2008;27:309-14. [PubMed]
- Ozdemir K, Altunkeser BB, Gök H, et al. Does the myocardial performance index affect pulmonary artery pressure in patients with mitral stenosis? A tissue Doppler imaging study. Echocardiography 2003;20:249-56. [PubMed]
- Karvounis HI, Papadopoulos CE, Zaglavara TA, et al. Evidence of left ventricular dysfunction in asymptomatic elderly patients with non-insulin-dependent diabetes mellitus. Angiology 2004;55:549-55. [PubMed]
- Yilmaz R, Seydaliyeva T, Unlü D, et al. The effect of left ventricular geometry on myocardial performance index in hypertensive patients. Anadolu Kardiyol Derg 2004;4:217-22. [PubMed]
- Bryg RJ, Pearson AC, Williams GA, et al. Left ventricular systolic and diastolic flow abnormalities determined by Doppler echocardiography in obstructive hypertrophic cardiomyopathy. Am J Cardiol 1987;59:925-31. [PubMed]
- Pearson AC, Labovitz AJ, Mrosek D, et al. Assessment of diastolic function in normal and hypertrophied hearts: comparison of Doppler echocardiography and M-mode echocardiography. Am Heart J 1987;113:1417-25. [PubMed]
- Sulica R, Teirstein AS, Kakarla S, et al. Distinctive clinical, radiographic, and functional characteristics of patients with sarcoidosis-related pulmonary hypertension. Chest 2005;128:1483-9. [PubMed]
- Handa T, Nagai S, Miki S, et al. Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest 2006;129:1246-52. [PubMed]


