Epithelial-mesenchymal transition-induced metastasis could be a bait for natural killer cells
Recent research has shown that several mechanisms induce tumor progression and a novel cause is the escape of tumor cells from immune surveillance (1). Natural killer (NK) cells play an important role in immunosurveillance against cancer cells (2), and the immunosurveillance by NK cells involves NK cell-mediated tumor cytotoxicity, which depends on the balance between NK cell-activating and -inhibiting ligands expressed in tumor cells (3). Both major histocompatibility complex (MHC) class I chain-related molecule A and B (MICA/B) and UL16-binding proteins (ULBPs) are NK cell group 2 member D (NKG2D) ligands, which activate NK cells via NKG2D-NKG2D ligand interaction (4,5). Moreover, recent research shows that cell adhesion molecule 1 (CADM1) is an NK cell-activating ligand, which engages in cytotoxic and regulatory T cell-associated molecule (CRTAM) receptor on NK cells (6). In addition, MHC class I has been recognized as a classical NK cell-inhibitory ligand, which engages killer cell immunoglobulin-like receptor (KIR) (7). Additionally, epithelial cadherin (E-cadherin) has been identified as an inhibitory ligand, which engages the killer lectin-like receptor G1 (KLRG1) (8). Several ligands recognized by NK cells have been identified as prognostic factors for patients with non-small cell lung cancer (NSCLC). The overexpression of MICA/B predicts improved clinical outcome (9), whereas KIR overexpression predicts poor prognosis in NSCLC (10), suggesting that NK cells are critical for immunosurveillance against tumor progression in NSCLC. These findings provide us the strategy for enhancing NK cell-activating receptor-ligand interaction while attenuating NK cell-inhibitory one in order to boost NK cell-mediated antitumor response in patients with NSCLC.
Epithelial-mesenchymal transition (EMT) is the event in which epithelial cells downregulate epithelial markers (e.g., E-cadherin, cytokeratin) but upregulate mesenchymal markers (e.g., N-cadherin, vimentin, and snail), resulting in tumor progression via dissolution of cell-cell adhesions and breaching of the basement membrane to facilitate migration and invasion (11). In addition, recent research showed that EMT led tumor cells to develop a resistant phenotype against anticancer regents as well as host immunosurveillance (12). EMT-associated secretory phenotype was reported to be predictive of poor clinical outcome, which suggested that EMT affects tumor progression, including metastatic activity of NSCLC (13). Thus, EMT is critical for metastasis; however, the majority of tumor cells in metastatic processes are successfully eradicated by host immunity (14), which suggests that metastatic cells may be vulnerable to host immunosurveillance after EMT.
Recently, Chockley et al. published important findings addressing the potential role of NK cells in blocking EMT-induced metastasis in lung cancer. In this study, EMT-reduced E-cadherin and EMT-induced CADM1 led to increased susceptibility to NK cell-mediated cytotoxicity in lung cancer cells, whereas EMT-induced NKG2D ligands did not enhance NK killing. Interestingly, they showed that depletion of NK cells allowed spontaneous metastasis without affecting primary tumor growth in vivo using several mouse models (15). These findings demonstrated the presence of NK cell-dependent metastasis-specific immunosurveillance in host immunity. To explain why NK cells only helped in prevention of metastasis, they demonstrated the role of transforming growth factor (TGF)-β-induced EMT (15). TGF-β is known as both an EMT-inducer (16) and an immunosuppressive cytokine for NK cells (17). The tumor microenvironment is recognized as TGF-β rich (18), which suggests that EMT is induced, whereas NK cell function is inactivated in the primary site, resulting in attenuated NK cell-mediated cytotoxicity. However, TGF-β levels in circulation or the site of micrometastasis may be lower than that in the primary site, which might lead to the enhancement of NK cell-mediated cytotoxicity via both upregulation of EMT-induced CADM1 and downregulation of EMT-reduced E-cadherin without dysfunction of NK cells in NSCLC cells (Figure 1).
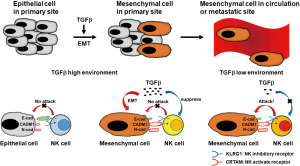
Several studies, including ours, have reported that modulation of the expression of NK cell-activating or -inhibiting ligands by artificial stimuli-induced DNA stress, such as radiation or cytotoxic chemotherapeutics, could affect NK cell-mediated cytotoxicity against tumor cells (3,9,19-21). This suggests that the enhancement of the activating ligand or attenuation of the inhibitory ligand is a promising strategy for cancer therapy. Moreover, EMT is an inevitable step for tumor progression, which implies that EMT-induced modulation of NK killing (15) has much impact to understand how tumor metastasis can be prevented via host immunity. Further, the authors showed CADM1 overexpression predicted improved clinical outcome in patients with NSCLC (15), which was consistent with previous reports, which showed that the overexpression of NKG2D ligand predicted improved prognosis in several types of cancer, including NSCLC (9,22). Presently, the NK cell-rerated ligand with the greatest effect on the survival of patients with NSCLC is unknown.
In addition, the authors have mentioned future research directions. We do not know the step of the metastatic cascade, which is critical for the prevention of metastasis by NK cells (15). To evaluate this task, in vivo live imaging tracking immune responses (23) might be useful. Moreover, investigation of the expression of E-cadherin and CADM1 among radiotherapy, cytotoxic chemotherapy, molecular target therapy, or immune checkpoint blocking therapy in patients with NSCLC should be conducted to understand the roles of E-cadherin and CADM1 in cancer treatment. Although a recent hot topic in cell therapy against cancer is chimeric antigen receptor (CAR) engineering T cells (24), it is also documented that NK cells with CAR engineering (CAR-NK) can successfully attack specific to solid tumor cells (25). To enhance the killing activity of NK cells specific to metastatic tumor cells with EMT, the CAR-NK strategy is quite promising.
Theoretically, EMT can be easy to excluded by NK cells in both the primary and metastatic sites, but Chockley et al. showed that NK cells could not affect tumor cells in the primary site (15). Therefore, we require a different strategy for treating the primary site and preventing metastasis. Surgical removal or radiation therapy for primary sites, combined with boosting NK cell function by CAR engineering or immune checkpoint inhibitor targeting KIR for targeting metastasis-specific immunosurveillance and preventing metastasis might be a promising combination strategy for NSCLC treatment.
Acknowledgements
The authors thank Editage for the language editing.
Funding: This work was supported by Japanese Society for the Promotion of Science (JSPS) Kakenhi Grants (grant number 16K10696).
Footnote
Conflicts of Interest: Dr. M Nakata received research funding from Kyowa Kirin, Taiho Pharma, Ono Pharma, and Nihon Medi-Physics for research outside the scope of the submitted work. The other authors have no conflicts of interest to declare.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Lanier LL. A renaissance for the tumor immunosurveillance hypothesis. Nat Med 2001;7:1178-80. [Crossref] [PubMed]
- Cerwenka A, Baron JL, Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc Natl Acad Sci U S A 2001;98:11521-6. [Crossref] [PubMed]
- Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999;285:727-9. [Crossref] [PubMed]
- Cosman D, Mullberg J, Sutherland CL, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity 2001;14:123-33. [Crossref] [PubMed]
- Boles KS, Barchet W, Diacovo T, et al. The tumor suppressor TSLC1/NECL-2 triggers NK-cell and CD8+ T-cell responses through the cell-surface receptor CRTAM. Blood 2005;106:779-86. [Crossref] [PubMed]
- Anfossi N, Andre P, Guia S, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity 2006;25:331-42. [Crossref] [PubMed]
- Schwartzkopff S, Grundemann C, Schweier O, et al. Tumor-associated E-cadherin mutations affect binding to the killer cell lectin-like receptor G1 in humans. J Immunol 2007;179:1022-9. [Crossref] [PubMed]
- Okita R, Yukawa T, Nojima Y, et al. MHC class I chain-related molecule A and B expression is upregulated by cisplatin and associated with good prognosis in patients with non-small cell lung cancer. Cancer Immunol Immunother 2016;65:499-509. [Crossref] [PubMed]
- He Y, Bunn PA, Zhou C, et al. KIR 2D (L1, L3, L4, S4) and KIR 3DL1 protein expression in non-small cell lung cancer. Oncotarget 2016;7:82104-11. [Crossref] [PubMed]
- Tarin D, Thompson EW, Newgreen DF. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res 2005;65:5996-6000; discussion 6000-1. [Crossref] [PubMed]
- Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol 2017;14:611-29. [Crossref] [PubMed]
- Reka AK, Chen G, Jones RC, et al. Epithelial-mesenchymal transition-associated secretory phenotype predicts survival in lung cancer patients. Carcinogenesis 2014;35:1292-300. [Crossref] [PubMed]
- Eyles J, Puaux AL, Wang X, et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J Clin Invest 2010;120:2030-9. [Crossref] [PubMed]
- Chockley PJ, Chen J, Chen G, et al. Epithelial-mesenchymal transition leads to NK cell-mediated metastasis-specific immunosurveillance in lung cancer. J Clin Invest 2018;128:1384-96. [Crossref] [PubMed]
- Miettinen PJ, Ebner R, Lopez AR, et al. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol 1994;127:2021-36. [Crossref] [PubMed]
- Ghiringhelli F, Menard C, Terme M, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med 2005;202:1075-85. [Crossref] [PubMed]
- Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet 2001;29:117-29. [Crossref] [PubMed]
- Karre K, Ljunggren HG, Piontek G, et al. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 1986;319:675-8. [Crossref] [PubMed]
- Gasser S, Orsulic S, Brown EJ, et al. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005;436:1186-90. [Crossref] [PubMed]
- Okita R, Wolf D, Yasuda K, et al. Contrasting Effects of the Cytotoxic Anticancer Drug Gemcitabine and the EGFR Tyrosine Kinase Inhibitor Gefitinib on NK Cell-Mediated Cytotoxicity via Regulation of NKG2D Ligand in Non-Small-Cell Lung Cancer Cells. PLoS One 2015;10. [Crossref] [PubMed]
- Watson NF, Spendlove I, Madjd Z, et al. Expression of the stress-related MHC class I chain-related protein MICA is an indicator of good prognosis in colorectal cancer patients. Int J Cancer 2006;118:1445-52. [Crossref] [PubMed]
- Ishii M, Egen JG, Klauschen F, et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 2009;458:524-8. [Crossref] [PubMed]
- Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med 2018;378:439-48. [Crossref] [PubMed]
- Uherek C, Tonn T, Uherek B, et al. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood 2002;100:1265-73. [PubMed]


