Efficacy and safety of low-dose clopidogrel after 12-month dual antiplatelet therapy for patients having drug-eluting stent implantation
Introduction
The widespread use of drug-eluting stents (DESs) for coronary heart disease has significantly reduced the risk of in-stent restenosis (ISR) but along with a tendency in increasing the risk of stent thrombosis (ST), which is associated with a mortality rate of 20% to 45% (1,2). To prevent ST after percutaneous coronary intervention (PCI) with DES, dual antiplatelet therapy (DAPT) with aspirin (100 mg/d) and clopidogrel (75 mg/d) for at least 12 months has become a class I recommendation in the treatment guidelines. Premature discontinuation of DAPT has also been regarded as a risk factor for ST (3-5). Following DAPT, monotherapy with either aspirin or clopidpgrel (when aspirin is contraindicated or not tolerated) is recommended for long-term use. However, there is serious concern about complications like bleeding and gastrointestinal intolerance during long-term, usually life-long, antiplatelet administration.
Clopidogrel, the key component of DAPT, is a prodrug modified through oxidation in liver and its active metabolite is selectively and irreversibly combined to platelet adenosine diphosphate receptors, thus inhibiting platelet aggregation. The efficacy and safety of clopidogrel in the secondary prevention of cardiovascular and cerebrovascular diseases have been confirmed by several large scale trials, such as CAPRIE, CURE, and COMMIT (6-8), most of which were carried out with the original clopidogrel (Plavix®, Sanofi-Synthelabo, France). However, the high cost of clopidogrel has been known as a factor in the premature discontinuation of therapy, resulting in an increase of major adverse cardiac events (MACEs) (9). In China, one alternative generic clopidogrel (Talcom®, Shenzen Salubris) has been released. It is much cheaper and has a lower content of 25 mg per tablet. As a result, the price advantage encouraged both doctors and patients to switch to the alternative clopidogrel product with a lower dose (Talcom®, 25-50 mg/d) from the innovator clopidogrel (Plavix®, 75 mg/d) that had been formerly received. Consequently, in the current clinical practice in China, DES-PCI patients after 12-month DAPT may have a choice to continue their antiplatelet therapy with any of the three drugs: aspirin (100 mg/d), clopidogrel (Plavix®, 75 mg/d) and clopidogrel (Talcom®, 25 mg/d).
This retrospective study aimed to compare the efficacy and safety among the three antiplatelet medications: low-dose clopidogrel (Talcom®, 25 mg/d), clopidogrel (Plavix®, 75 mg/d) and aspirin (100 mg/d) which were used in patients who had undergone DES implantation and completed 12-month DAPT.
Materials and methods
Study population and data collection
The clinical data were retrospectively collected between September 2008 and May 2013 from the 796 consecutive patients who had undergone PCI three years ago and completed 12-month DAPT at the Cardiac Center of The First Affiliated Hospital of Sun Yat-Sen University. The choice of DES was at operators’ discretion and PCI was performed using standard techniques. Of these 796 patients we retrieved the records of long-term antiplatelet regimens. They had aspirin 100 mg/d, clopidogrel (Plavix®) 75 mg/d, or clopidogrel (Talcom®) 25 mg/d after 12-month DAPT. We also reviewed their demographic, clinical, angiographic and procedural characteristics at baseline, including age, sex, body mass index (BMI), systolic and diastolic blood pressure (SBP and DBP), history of hypertension and stroke, lipid profile and kidney function. We excluded the patients who had hypoxic encephalopathy, malignancies, or chronic hemodialysis (HD), were taking warfarin or single antiplatelet therapy, or had been transferred to other hospitals after a successful PCI at the first affiliated hospital of Sun Yat-sen University. The protocol was approved by the hospital ethics committee.
Definitions and follow-up
The primary endpoint for outcome efficacy was MACE defined as a combined incidence of cardiac death, myocardial infarction, urgent target vessel revascularization (coronary bypass surgery or PCI) due to myocardial infarction. Mortality data of cardiac death were collected from the medical records of the patients. The definition of myocardial infarction was development of pathologic Q waves (≥30 ms in duration and ≥0.1 mV in depth) in ≥2 contiguous precordial leads or ≥2 adjacent limb leads, or elevation of creatine kinase isoenzyme MB (CK-MB) ≥2 times the upper limit of normal. On follow-up coronary angiography (CAG), target lesion revascularization was considered clinically driven if prompted by symptoms or signs consistent with myocardial ischemia or if lesion diameter stenosis was more than 70% at follow-up.
The safety endpoints of the study were incidences of minor/major bleeding, gastrointestinal trouble, drug discontinuation at 36 months after DES placement. Major bleeding was defined as intracranial, intraocular, or retroperitoneal hemorrhage, clinically overt blood loss resulting in a decrease in hemoglobin of more than 3 g per deciliter, any decrease in hemoglobin of more than 4 g per deciliter, or transfusion of 2 or more units of packed red blood cells or whole blood (10). The gastrointestinal troubles referred to epigastric discomfort or pain, nausea, vomiting and diarrhea.
Statistical analysis
The data are presented as mean ± SD or frequencies (%). Categorical data were compared with the chi-squared test or Fisher’s exact test when cell values were less than 5. Multiple testing of continuous data were analyzed using one-way ANOVA and post hoc Bonferroni’s test since the assumption of linearity and normal distribution of residuals were achieved. For categorical data, multiple testing was conducted with the chi-squared test followed by Bonferroni’s posttest. Survival analysis was performed by the Kaplan-Meier method. Differences in the cumulative adverse cardiac events were assessed with the log-rank test, which allowed the calculation of odds ratio (OR) [95% confidence intervals (CI)] associated with clopidogrel (25 mg/d) group. A P value <0.05 was considered to indicate statistical significance. All data were analyzed using SPSS version 16.0 for Windows (SPSS Inc, Chicago, IL, USA).
Results
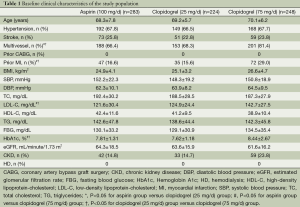
Baseline characteristics of patients are shown in Table 1. Of the total 796 patients, 41 patients were further excluded for lack of data (n=11), and cannot follow-up in our hospital (n=30). Among the 755 patients included in the analysis, 283 were in aspirin (100 mg/day) group, 224 clopidogrel (25 mg/day) group and 248 clopidogrel (75 mg/day) group. The mean follow-up duration was 36±2.4 months after DES-PCI, with 12-month DAPT completed. The age, history of hypertension and stroke, BMI, SBP, DBP, high-density lipoprotein-cholesterol (HDL-C), triglycerides (TG), fasting blood glucose (FBG), estimated glomerular filtration rate (eGFR), chronic kidney disease (CKD) at baseline did not differ significantly among the three groups. However, there were significantly more patients with multivessel lesions, prior MI, hemoglobin A1c (HbA1c) in clopidogrel (75 mg/day) group than in clopidogrel (25 mg/day) and aspirin (100 mg/day) groups (P>0.05 for both comparisons). Moreover, clopidogrel (75 mg/day) group had a significantly higher level of low-density lipoprotein cholesterol (LDL-C) than the other two groups (P<0.05). No significant difference existed among the three groups regarding total and high-density lipoprotein cholesterol (HDL-C).
Full table
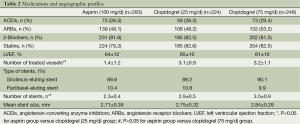
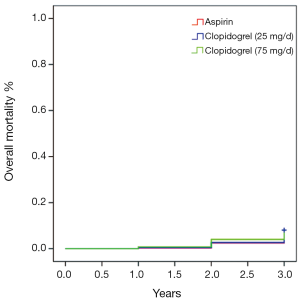
Table 2 shows medication profiles at discharge and the angiographic features of the patients included in the present study. The medications of Angiotensin-Converting Enzyme Inhibitors (ACEIs), Angiotensin Receptor Blockers (ARBs) and statins use, as well as left ventricular ejection fraction, showed no difference in frequency among the three groups. The mean stent size and DES type used did not differ among the three groups, either. However, the numbers of treated vessel and stent were significantly lower in the aspirin treatment group than in the two clopidogrel treatment groups (P<0.05), but not significantly different between the latter two groups (P=0.16). As shown in Table 3, there were no significant difference in the overall composite incidence of cardiac death, myocardial infarction and target vessel revascularization in the three groups at three years after PCI. Specifically, 25 deaths occurred in aspirin (100 mg/day) group, 18 in clopidogrel (25 mg/day) group and 18 in clopidogrel (75 mg/day) group, giving a 3-year mortality rate of 9.9%, 8% and 7.3% respectively (P>0.05) (Figure 1). The cumulative incidence of overall mortality presented in Figure 1 shows the Kaplan-Meier survival curve. There was no significant diverge in the overall mortality rate among the three groups at three years. Table 4 shows the safety endpoints among the three groups. The bleeding (especially minor bleeding), gastrointestinal trouble, drug discontinuation and any blood transfusion in aspirin group were markedly higher than in the other two clopidogrel treatment groups (P<0.05). Moreover, compared with clopidogrel (75 mg/d) group, clopidogrel (25 mg/d) group had a lower event rate in terms of minor bleeding and gastrointestinal trouble.
Full table

Full table

Full table
Discussion
This retrospective study assessed the efficacy and safety of long term (approximately two years) administration of standard and low-dose clopidogrel (25 mg/d) and aspirin (100 mg/d) after 12-month DAPT in real world patients who had undergone DES implantation. The main findings of this study are as follows: (I) in daily practice, there were no significant differences regarding the composite endpoint of cardiac death, myocardial infarction and urgent target vessel revascularization among patients receiving single treatment of different doses of clopidogrel and aspirin three years after DES implantation; (II) low-dose clopidogrel regime (Talcom®, 25 mg/d) resulted in fewer bleeding events, better gastrointestinal tolerance and medicine compliance than standard clopidogrel (Plavix®, 75 mg/d) and aspirin (100 mg/d) ones.
The delayed healing following DES placement (and therefore the optimal duration of anti-platelet therapy) has been the subject of much recent debate and an American advisory board has strongly recommended thienopyridine treatment for at least 12 months in all patients after DES implantation (9). After a standard duration of DAPT (12 months), the patients may have the option of aspirin or cliopidogrel (75 mg/d) for long-term single antiplatelet treatment, as there is no doubt that monotherapy of these agents are effective following DAPT in patients after DES-PCI (4). However, increasingly widespread use of the generic clopidogrel (Talcom®) in China has raised doubts about the pharmacokinetic and pharmacodynamic action as well as the tolerability of the copy form drug. Investigations were performed to clarify these doubts (11-13). One study failed to demonstrate significant differences either in the measure or in the tolerability of platelet aggregation between the two forms at the same dosage in healthy volunteers (11). On the contrary, our data showed that, during 3-year follow-ups, there were no significant differences in MACE and bleeding events between standard clopidogrel (75 mg/d) and low-dose clopidogrel (25 mg/d) after 12-month DAPT, indicating low-dose generic clopidogrel may serve as an effective alternative of the standard-dose original agent while it incurs a significantly lower cost. Moreover, our study revealed a significant lower drug discontinuation rate in the patients taking low-dose clopidogrel than those taking the other two agents, suggesting that much less cost of low-dose clopidogrel may be associated with better treatment compliance. A multicenter, prospective, randomized trial showed that, in patients undergoing selected PCI, there were no significant differences in MACE between domestic clopidogrel (Talcom®) and Plavix® (12). Moreover, another randomized trail evaluated the efficacy and safety of 50 mg clopidogrel in Japanese patients who underwent DES implantation (13). During follow-up, no significant difference in cardiac death, myocardial infarction or ST was observed in low dose clopidogrel group compared with standard clopidogrel group, as well as side effects. This result can attribute to ethnic difference. A previous study showed that maintenance doses of some drugs differed between Asian patients and Caucasian patients (14,15). Similarly, Fukushima et al. reported a similar antiplatelet effect between 200 mg ticlopidine and 50 mg clopidogrel in Japanese patients, and 50 mg clopidogrel is much lower than the “standard” dosage (75 mg) for Caucasian patients (16). Taken together, given the potential benefit of lower incidence of side effects that low dose clopidogrel maintenance may bring about, as well as the ethnic difference, a lower maintenance dose of clopidogrel may be considered appropriate in Chinese patients after 12-month DAPT.
Studies have identified, besides the above antiplatelet agents, stent underexpansion, dissection, long stent length, and residual stenosis at stent edges as procedure-related risk factors of ST (17-19). Thus, besides optimal medication, it is important to achieve optimal stent deployment to prevent ST. Roy et al. have demonstrated a lower ST rate after DES implantation under intravascular ultrasound guidance when compared with angiographic guidance (20). Premature discontinuation or ineffectiveness of antiplatelet drugs predisposes the patients to ST (5). Analysis of the Dutch ST registry (21,009 patients and a total of 31,065 stents) showed that discontinuation of antiplatelet therapy with clopidogrel was a strong independent predictor of ST (21). Cessation of clopidogrel in the first 30 days after PCI enhanced the hazard ratio for ST to 36.5 (95% CI: 8.0 to 167.8), and the lack of clopidogrel therapy between 30 days and six months was also linked to a significantly increased risk of ST (hazard ratio 4.6, 95% CI: 1.4 to 15.3) (21). Similarly, the absence of aspirin therapy was also independently related to ST (21). In other words, the low-dose clopidogrel therapy may be applicable to those patients that procedure related risk factors of ST were under well-controlled, for example, by the use of intravascular ultrasound-guided DES implantation.
There were some limitations to our analysis. Firstly, our study was an observational one. It was possible that confounding factors could have accounted for the observed differences. Secondly, the relatively short follow-up duration did not allow us to monitor the overall effect of low-dose clopidogrel on the whole progression of cardiovascular disease. Further clinical trials in large cohorts of patients are necessary to compare antiplatelet agents. Thirdly, we did not assess plasma levels of the active metabolites of clopidogrel either, because the authorization of this retrospective study did not allow us to take extra blood samples from the patients for assessment of the clopidogrel plasma levels or platelet function.
Conclusions
This retrospective study shows safety and efficacy of 25 mg maintenance dose of clopidogrel after 12-month DAPT in Chinese patients undergoing DES implantation, justifying its advantage of low-cost over the original product. However, this conclusion should be interpreted with caution before large-scale randomized trials come to a definite one comparing doses of 25 and 75 mg clopidogrel in Chinese patients undergoing coronary stent implantation after DAPT.
Acknowledgements
This study was supported by National Nature Science Foundation of China (Grant No. 81270296) and Science and Technology Planning Project of Guangdong Province in China (2010B080701105). We thank all the cardiology fellows, catheterization laboratory staff members and nurses who were an integral part of the study at the First Affiliated Hospital, Sun Yat-sen University.
Disclosure: The authors declare no conflict of interest.
References
- Minha S, Pichard AD, Waksman R. In-stent restenosis of drug-eluting stents. Future Cardiol 2013;9:721-31. [PubMed]
- Haine SE, Vrints CJ. Stent thrombosis with drug-eluting and bare-metal stents. Lancet 2012;380:646-author reply 646-7. [PubMed]
- Park SJ, Park DW, Kim YH, et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N Engl J Med 2010;362:1374-82. [PubMed]
- Yu X, Chen F, He J, et al. Duration of dual antiplatelet therapy after implantation of the first-generation and second-generation drug-eluting stents. Coron Artery Dis 2013;24:217-23. [PubMed]
- Rossini R, Capodanno D, Lettieri C, et al. Prevalence,predictors,and long-term prognosis of premature discontinuation of oral antiplatelet therapy after drug eluting stent implantation. Am J Cardiol 2011;107:186-94. [PubMed]
- Gent M. The CAPRIE trial: culmination of the preregistration program for clopidogrel. Clopidogrel versus aspirin in patients at risk of ischaemic events. Clopidogrel versus aspirin in patients at risk of ischaemic events. Semin Thromb Hemost 1999;25 Suppl 2:1-2. [PubMed]
- Gerschutz GP, Bhatt DL. The CURE trial: using clopidogrel in acute coronary syndromes without ST-segment elevation. Cleve Clin J Med 2002;69:377-8, 380, 382 passim. [PubMed]
- Bode C. Therapy of acute mayocardial infarction: COMMIT (Clopidogrel and Metoprolol Infarction Trial). Internist (Berl) 2006;47:764-6. [PubMed]
- Grines CL, Bonow RO, Casey DE Jr, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. J Am Dent Assoc 2007;138:652-5. [PubMed]
- Quinlan DJ, Eikelboom JW, Goodman SG, et al. Implications of variability in definition and reporting of major bleeding in randomized trials of oral P2Y12 inhibitors for acute coronary syndromes. Eur Heart J 2011;32:2256-65. [PubMed]
- Kim SD, Kang W, Lee HW, et al. Bioequivalence and tolerability of two clopidogrel salt preparations, besylate and bisulfate: a randomized, open-label, crossover study in healthy Korean male subjects. Clin Ther 2009;31:793-803. [PubMed]
- Meng K, Lü SZ, Zhu HG, et al. Use of tailored loading-dose clopidogrel in patients undergoing selected percutaneous coronary intervention based on adenosine diphosphate-mediated platelet aggregation. Chin Med J (Engl) 2010;123:3578-82. [PubMed]
- Ohkubo K, Fujimoto Y, Iwata Y, et al. Efficacy and safety of low-dose clopidogrel in Japanese patients after drug-eluting stent implantation: a randomized pilot trial. Heart Vessels 2014;29:1-6. [PubMed]
- Fukushima K, Kobayashi Y, Okuno T, et al. Incidence of side-effects of ticlopidine after sirolimus-eluting stent implantation. Circ J 2007;71:617-9. [PubMed]
- Nakayama A, Morita H, Ando J, et al. Adverse cardiovascular outcomes associated with concurrent use of clopidogrel or ticlopidine and proton-pump inhibitors in patients undergoing percutaneous coronary intervention. Heart Vessels 2013;28:292-300. [PubMed]
- Fukushima K, Kobayashi Y, Kitahara H, et al. Antiplatelet effect of 50-mg maintenance dose of clopidogrel compared to 200 mg ticlopidine: a preliminary study. Heart Vessels 2010;25:41-4. [PubMed]
- Fujii K, Carlier SG, Mintz GS, et al. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study. J Am Coll Cardiol 2005;45:995-8. [PubMed]
- Moreno R, Fernández C, Hernández R, et al. Drug-eluting stent thrombosis: results from a pooled analysis including 10 randomized studies. J Am Coll Cardiol 2005;45:954-9. [PubMed]
- Brodie B, Pokharel Y, Garg A, et al. Predictors of early, late, and very late stent thrombosis after primary percutaneous coronary intervention with bare-metal and drug-eluting stents for ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2012;5:1043-51. [PubMed]
- Roy P, Steinberg DH, Sushinsky SJ, et al. The potential clinical utility of intravascular ultrasound guidance in patients undergoing percutaneous coronary intervention with drug-eluting stents. Eur Heart J 2008;29:1851-7. [PubMed]
- van Werkum JW, Heestermans AA, Zomer AC, et al. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol. 2009;53:1399-409. [PubMed]


