Analysis of mutations in 7 candidate genes for dextro-Transposition of the great arteries in Chinese population
Introduction
Transposition of the great arteries (TGA) is the most common cyanotic congenital heart lesion that presents in neonates. The hallmark of TGA is ventriculoarterial discordance, in which the aorta arises from the morphologic right ventricle and the pulmonary artery arises from the morphologic left ventricle. Despite its overall low prevalence, TGA is the most common etiology for cyanotic congenital heart disease (CHD) in the newborn (1). This lesion presents in 5-7% of all patients with CHD. The overall annual incidence is 20-30 per 100,000 live births, and inheritance is multifactorial. TGA is isolated in 90% of patients and is rarely associated with syndromes or extracardiac malformations. This congenital heart defect is more common in infants of diabetic mothers (2). The mortality rate in untreated patients is approximately 30% in the first week, 50% in the first month, and 90% by the end of the first year. With improved diagnostic, medical, and surgical techniques, the overall short-term and midterm survival rate exceeds 90% (3).
The exact etiology of TGA is still unknown. The controversy between environmental factors and genetic causes has been discussed for years. Some studies have postulated associated risk factors, such as gestational diabetes mellitus (4,5), maternal exposure to rodenticides and herbicides (6), and maternal use of antiepileptic drugs (7). However, significant progresses have been achieved in understanding the genetic causes of this disease. The genes being thought to be associated with the pathogenesis of TGA so far are the thyroid hormone receptor-associated protein-2 gene (MED13L) (8), nucleocytoplasmic shuttling protein gene (ZIC3) (9), the forkhead activin signal transducer 1 (FOXH1) (10), cryptic family 1 (CFC1) (11), growth differentiation factor 1 (GDF1) (11) and TGF-beta gene (NODAL), the latter five genes of which affect the LR-axis development (12). NK2 homeobox 5 (NKX2-5) are rarely involved in TGA pathogenesis (13). These genes are located in different chromosomes and their mutations only explain few clinical cases. The chromosomal region 22q11 was also suggested to be in the involvement of the pathogenesis of TGA (14).
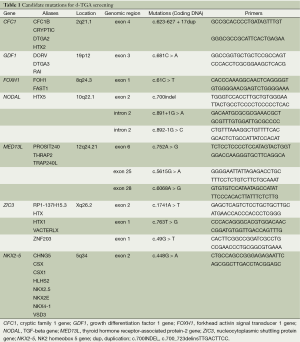
Since no genetic scan for TGA has been implemented in China, this study was initiated to evaluate whether aberrations in any of the reported 13 mutations (Table 1) in seven genes (MED13L, ZIC3, CFC1, NODAL, FOXH1, GDF1 and NKX2-5) could completely or in part be the genetic component involved in TGA in Chinese population. The basis for selecting these 13 mutations was their possible involvement in malformations of TGA. The purpose of the study was to perform mutational screening of these 13 mutations in d-TGA patients in China.
Full table
Patients and methods
Patients
Charts for patients with d-TGA in Guangdong General Hospital between Jan 1, 2000 and August 30, 2012 were reviewed. A total of 102 patients diagnosed by echocardiography, with age ranged from 10 days to 12-year-old were selected. The male-to-female ratio was 2.4:1. Fifty-four patients were TGA with intact ventricular septum (TGA/IVS), 31 patients were TGA with ventricular septum defect (TGA/VSD), 17 patients were combined with other complex situations as coarctation of aorta (CoA), pulmonary valve stenosis (Ps), single ventrical (SV) et al.
Genomic DNA extraction and mutational screening
After informed consent was obtained with approval by the Medical Ethical Review Committee of Guangdong General Hospital, constitutional DNA was prepared from peripheral blood with the PureGene DNA isolation kit (Gentra Systems, Minneapolis, MN, USA) according to the manufacturer’s recommendations. The sequences of 13 regions in MED13L, ZIC3, CFC1, NODAL, FOXH1, GDF1 and NKX2-5 were analyzed for mutations using direct sequencing analysis. Polymerase chain reaction (PCR) products were generated by exon flanking primers according to the reported 13 mutations in seven genes which might be associated with pathogenesis of TGA in human (Table 1).
Primers are given in Table 1. PCR reactions were performed in Eppendorf5332 PCR system in a total volume of 20 µL, using the mixture containing 10 µL GoTaq Green Master Mix (Promega), 10 µM forward primer, 10 µM reverse primer, 200 ng genomic DNA and 4 µL Nuclease-Free Water. The following amplification conditions was used: initial denaturation at 95 °C for 5 min, followed by 40 cycles including denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and elongation at 72 °C for 1 min. The procedure was completed by a final incubation at 72 °C for 5 min. The PCR products were then sent to Invitrogen Co. (Guangzhou, China) for sequencing on ABI 3730. Sequence analysis was carried out using Chromas.exe, and the homozygous and heterozygous alleles were scored manually.
Results
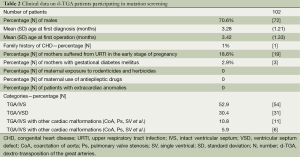
Patients’ clinical characteristics are given in Table 2. The percentage of males is 70.6% (72/102). The mean age at first diagnosis was 3.28 months (Standard Deviation, SD 1.21), while the mean age at first operation was 3.42 months (SD 1.33). 1% (n=1) of patients had family history of CHD, with the mother diagnosed of atrial septal defect (ASD). 18.6% (n=19) of mothers had suffered from upper respiratory tract infection in the early stage of pregnancy and 2.9% (n=3) were diagnosed with gestational diabetes mellitus. No mothers had been exposed to rodenticides and herbicides or took any antiepileptic drugs. All the patients were isolated TGA with the absence of extracardiac anomalies.
Full table
We sequenced candidate genomic regions where the 13 mutations were exactly located in MED13L, ZIC3, CFC1, NODAL, FOXH1, GDF1 and NKX2-5, and found none of the reported 13 mutations in those 102 Chinese d-TGA patients.
Discussion
TGA is the second most common congenital heart defect that causes problems in early infancy. Previous studies had focus on asymmetric cardiogenesis and several mutations have been implicated as the cause of this disease. However the total number of mutations detected so far is not sufficient to explain the high incidence of TGA. Besides, genetic screenings of TGA patients are rare in Chinese population. In this study we present the results of our efforts to screen a Chinese group of 102 d-TGA patients for the reported 13 mutations in MED13L, ZIC3, CFC1, NODAL, FOXH1, GDF1 and NKX2-5. Mutation analysis showed the absence of identical mutations in the 13 regions.
Two major hypotheses for TGA development have been suggested from the embryological point of view: an anomalous infundibular rotation and an aorticopulmonary septum anomaly (11). Nodal signaling pathway is responsible for early embryonic development, mesoderm and endoderm formation and left-right axis patterning in vertebrate embryos (15). The 6 genes of ZIC3, CFC1, NODAL, FOXH1, GDF1 and NKX2-5 are essential components of the NODAL signaling pathway, which are responsible for a subset of laterality defects. MED13L had been showed to affect nuclear receptor and cause severe defects during embryonic development (9).
For the pathogenesis of d-TGA, the involvement of 7 genes has already been discussed. In Muncke’s study (8), 97 patients with d-TGA were screened in MED13L for mutations and 6 intronic polymorphisms, 6 silent mutations, and 4 missense mutations were found, the mutation analyze strongly suggested 3 missense mutations (Glu251Gly, Arg1872His, and Asp2023Gly) were involved in the pathogenesis of d-TGA. De Luca and colleges (11) screened probands of seven families with isolated TGA and a family history of concordant or discordant CHD for mutations within the ZIC3, ACVR2B, LEFTYA, CFC1, NODAL, FOXH1, GDF1, CRELD1, GATA4 and NKX2-5 genes. Two missense mutations in FOXH1 (Pro21Ser) and ZIC3 (Gly17Cys), a splice site variant in NODAL (IVS2-1G/C) were detected. Also the role of EGF-CFC gene was investigated in gene-targeting studies in mice, knockout of CFC1 gene in mouse results in laterality defects and complex cardiac malformations including TGA (16,17). Two distinct mutations of CFC1 were identified in three independent patients with laterality defects and d-TGA in Bamford’s research (18). GDF1 mutated in a variety of CHD including TGA (19), and NKX2-5mutations have also previously been identified in patients with CHD including septal defects, but they are very rarely detected in TGA (20). These results confirm that genetic heterogeneity of this congenital heart defect is related to the heterogeneity of the mechanisms that finally produce the same phenotype.
In our study, none of the 13 mutations was found in 102 Chinese patients. There could be several possible explanations to the lack of mutations in this study. First of all, we did not examined the whole sequence of the seven genes and only checked thirteen mutations in them, thus we cannot exclude the possibility that certain mutations were missed by our direct sequencing approach. Secondly, we may not be able to predict whether causal mutations could be found in TGA, and whether the mutations are loss- or gain-of-function mutations. If mutations of the former category were involved, they can account for the missing mutations according to the fact that loss-of-function mutations are located in the promoter region, or in other regulatory sequences affecting gene expression levels, it might not be detected by our mutation analysis approach. The same is true for deletions or insertions that interfere with any of the primer binding sites. Thirdly, some genes of L-R signaling pathways, which perturbing the development of human laterality and resulting in discordant orientation of great artery, like Lefty1 and Lefty2, were not tested. Moreover, the sample size might be not large enough. However, the results presented here strongly indicate that the 13 missense or deletion/insertion mutations in these seven genes may not be a common cause of TGA in Chinese population.
The low mutation frequency of MED13L, ZIC3, CFC1, NODAL, FOXH1, GDF1 and NKX2-5 in d-TGA patients underlines the heterogeneity of this disease, in which the candidate gene screening had limited success. Therefore, new approaches are required to identify genetic variants in TGA patients. Whole exome sequencing technology, with its high effectiveness to detect common and rare variations, identify the genes responsible for complex diseases, will be applied for sequencing the exome (1% of genome) to discover most of the diseases-related variations in exons in TGA.
This study highlights the fact that the underlying genetic etiology of d-TGA can be complex. Thirteen known mutations in MED13L, ZIC3, CFC1, NODAL, FOXH1, GDF1 and NKX2-5 may not be a common cause of d-TGA in Chinese population due to racial variation. Next-generation sequencing approaches including the whole exome sequencing technology could effectively identify disease-related genetic variants of d-TGA patients in China, which will be very helpful in understanding the pathogenesis of the disease and clinical diagnosis.
Conclusions
These reported 13 mutations may not be a common cause of d- TGA in Chinese population due to racial variation and genetic heterogeneity of TGA. New approaches including the whole exome sequencing technology are required to effectively identify genetic variants in TGA patients in China.
Acknowledgements
This work was supported by the National Key Technology Research and Development Program (2011BAI11B22), Medical Scientific Research Foundation of Guangdong Province (B2012002), and Guangdong Population and Family Planning Foundation (2012264). The authors are also grateful to the children and their parents for their willing participation in our study.
Disclosure: The authors declare no conflict of interest.
References
- Rao PS. Diagnosis and management of cyanotic congenital heart disease: part I. Indian J Pediatr 2009;76:57-70. [PubMed]
- Wren C, Birrell G, Hawthorne G. Cardiovascular malformations in infants of diabetic mothers. Heart 2003;89:1217-20. [PubMed]
- Aseervatham R, Pohlner P. A clinical comparison of arterial and atrial repairs for transposition of the great arteries: early and midterm survival and functional results. Aust N Z J Surg 1998;68:206-8. [PubMed]
- Becerra JE, Khoury MJ, Cordero JF, et al. Diabetes mellitus during pregnancy and the risks for specific birth defects: a population-based case-control study. Pediatrics 1990;85:1-9. [PubMed]
- Abu-Sulaiman RM, Subaih B. Congenital heart disease in infants of diabetic mothers: echocardiographic study. Pediatr Cardiol 2004;25:137-40. [PubMed]
- Loffredo CA, Silbergeld EK, Ferencz C, et al. Association of transposition of the great arteries in infants with maternal exposures to herbicides and rodenticides. Am J Epidemiol 2001;153:529-36. [PubMed]
- Okuda H, Nagao T. Cardiovascular malformations induced by prenatal exposure to phenobarbital in rats. Congenit Anom (Kyoto) 2006;46:97-104. [PubMed]
- Muncke N, Jung C, Rüdiger H, et al. Missense mutations and gene interruption in PROSIT240, a novel TRAP240-like gene, in patients with congenital heart defect (transposition of the great arteries). Circulation 2003;108:2843-50. [PubMed]
- Mégarbané A, Salem N, Stephan E, et al. X-linked transposition of the great arteries and incomplete penetrance among males with a nonsense mutation in ZIC3. Eur J Hum Genet 2000;8:704-8. [PubMed]
- Roessler E, Ouspenskaia MV, Karkera JD, et al. Reduced NODAL signaling strength via mutation of several pathway members including FOXH1 is linked to human heart defects and holoprosencephaly. Am J Hum Genet 2008;83:18-29. [PubMed]
- De Luca A, Sarkozy A, Consoli F, et al. Familial transposition of the great arteries caused by multiple mutations in laterality genes. Heart 2010;96:673-7. [PubMed]
- Mohapatra B, Casey B, Li H, et al. Identification and functional characterization of NODAL rare variants in heterotaxy and isolated cardiovascular malformations. Hum Mol Genet 2009;18:861-71. [PubMed]
- Reamon-Buettner SM, Borlak J. NKX2-5: an update on this hypermutable homeodomain protein and its role in human congenital heart disease (CHD). Hum Mutat 2010;31:1185-94. [PubMed]
- Melchionda S, Digilio MC, Mingarelli R, et al. Transposition of the great arteries associated with deletion of chromosome 22q11. Am J Cardiol 1995;75:95-8. [PubMed]
- Shen MM. Nodal signaling: developmental roles and regulation. Development 2007;134:1023-34. [PubMed]
- Gaio U, Schweickert A, Fischer A, et al. A role of the cryptic gene in the correct establishment of the left-right axis. Curr Biol 1999;9:1339-42. [PubMed]
- Yan YT, Gritsman K, Ding J, et al. Conserved requirement for EGF-CFC genes in vertebrate left-right axis formation. Genes Dev 1999;13:2527-37. [PubMed]
- Bamford RN, Roessler E, Burdine RD, et al. Loss-of-function mutations in the EGF-CFC gene CFC1 are associated with human left-right laterality defects. Nat Genet 2000;26:365-9. [PubMed]
- Karkera JD, Lee JS, Roessler E, et al. Loss-of-function mutations in growth differentiation factor-1 (GDF1) are associated with congenital heart defects in humans. Am J Hum Genet 2007;81:987-94. [PubMed]
- McElhinney DB, Geiger E, Blinder J, et al. NKX2.5 mutations in patients with congenital heart disease. J Am Coll Cardiol 2003;42:1650-5. [PubMed]


