Performance of mass spectrometric identification of bacteria and yeasts routinely isolated in a clinical microbiology laboratory using MALDI-TOF MS
Introduction
Infectious disease is the most common clinical disease, and fast and accurate diagnosis is paramount to control infection. Traditionally, the identification of pathogenic bacteria and yeast has relied on conventional culture, isolation and biochemical identification methods (1), the latter of which can be complicated and requires prolonged turnaround times. In addition, the biochemical characteristics of atypical bacteria are often difficult to identify, which causes concern with regards to choosing effective antibiotic therapy in a timely manner. Empirical application of broad-spectrum antibiotics to treat unidentified pathogenic bacteria and yeast leads to the emergence of more resistant strains, which further increases the effectiveness and costliness of clinical treatment. Therefore, identification of clinical pathogenic bacteria and yeast not only promotes rapid diagnosis and treatment disease, but it also helps reduce both the emergence of drug-resistant strains and the costs associated with drug-resistant strains present in the clinic.
While techniques in molecular biology (i.e., ribosomal gene sequence analysis, real-time quantitative PCR, gene chips) (2) provide rapid methods for identification of bacteria and yeast, their high cost and complexity often prohibit these molecular techniques from being applied to routine testing in the clinical microbiology laboratory. In order to meet clinical needs, it is urgent to establish a rapid diagnostic method for routinely identifying pathogenic bacteria and yeast. Matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS) is a new platform that is being increasingly applied to the clinical microbiology laboratory for use of rapid and accurate identification of pathogenic bacteria and yeast (3-16). The present study was conducted to explore the accuracy and feasibility of MALDI-TOF-MS in identifying clinically isolated bacteria and yeast.
Materials and methods
Bacterial and yeast isolates
All isolates were prospectively recovered over a 14-week period from various clinical specimens (such as blood, cerebrospinal fluid, urine, pus, biopsy, swab from any site of the body, pleural effusion, hydroperitoneum, respiratory tract, and wound specimens) sourced from different medical departments. Isolate duplicates (i.e., from the same patient) were discarded. The isolates were recovered after aerobic and anaerobic incubation of clinical specimens on 5% sheep-blood and chocolate agar media (bioMérieux). After semi-automated Gram staining (bioMérieux) and determination of catalase and oxidase activities, isolates were identified by using either the Vitek 2-Compact system (bioMérieux) or an appropriate API identification strip (bioMérieux). In parallel, a single colony of a (sub) culture was directly deposited on a MALDI-TOF plate (VITEK® MS, bioMérieux). Technicians performing one method of identification were blind to the results obtained from the other method.
Mass spectrometry
Technical training
Three technicians were trained for sample and slide preparation by performing three slides of 48 deposits with duplicate deposits per isolate during three independent days (one slide per day). Mucoid and rough isolates were included only in the third slide performed by each operator. A proficiency test using 16 strains with single deposits was passed by each technician.
Plate preparation
The disposable plate preparation was performed with the Vitek® MS preparation station software to link sample information to the mass spectrometer using the single-use FlexiMass MALDI target plates, supplied in a 48-well microscope slide format, divided into three acquisition groups of 16 spots, and by smearing the bacteria or yeast directly onto the plate (mostly one colony/deposit). The preparations for bacteria were overlaid with 1 µL of ready-to-use ɑ-cyano-4-hydroxycinnamic acid (CHCA) matrix (bioMérieux) and air dehydrated for 1 to 2 min at room temperature. For yeast preparations were lysed with 0.5 µL 25% formic acid. After drying completely at room temperature (1 to 2 min), 1 µL of CHCA matrix (bioMérieux) was applied to the spot, which was also allowed to dry completely (1 min). As recommended by the manufacturer’s instructions, the Escherichia coli ATCC 8739 strain, used as a calibrator and internal ID control, was inoculated on the calibration spots of each acquisition group (small spot in the middle of each acquisition group). Each bacterial isolate had been tested with a unique deposit.
Generation of mass spectra
Mass spectra were generated with a Vitek® MS Axima Assurance mass spectrometer (bioMérieux) in positive linear mode at a laser frequency of 50 Hz with an acceleration voltage of 20 kV and an extraction delay time of 200 ns. For each spectrum, 500 shots in 5-shot steps from different positions of the target spot (automatic mode) were collected by the mass spectrometer operating in conjunction with the Acquisition Station software (Vitek® MS version 2.0). Measured mass spectra ranged from 2,000 to 20,000 Da.
MS identification
For each bacterial or yeast sample, mass fingerprints were processed by the compute engine and the advanced spectrum classifier (ASC) algorithm associated with the Vitek® MS system, which then automatically identifies the organism by comparing the characteristics of the spectrum obtained (presence and absence of specific peaks) with those of the typical spectrum of each claimed species contained in the database.
The ASC algorithm compared the generated spectra to the expected spectrum of each organism or organism group of the database to provide identification. A percent probability, which represents the similarity in terms of presence/absence of specific peaks between the generated spectrum and the database spectra, was calculated by the algorithm. Isolates with scores from 60% to 99.9% with a single organism choice were considered a good identification. For isolates with probability scores >60% and a choice of 2-4 organisms, a genus level identification was recorded if all choices were within the same genus. However, no valid identification was recorded if the organism choices were of multiple genera. Scores of <60% were considered to have no valid identification.
When a human error or a poor-quality deposit occurred (including the warning messages “bad spectrum”, “not enough peaks”, “too many peaks”, and “too much background noise” or in the case of calibration/control failure), the isolates were retested with a single deposit and the second result used for analysis. For informative purposes, samples with “no ID” or “mis-ID” first-spot results were secondarily retested with a single spot.
Criteria for identification of isolates
Accurate identification of isolates using the Vitek 2-Compact system or the API system was confirmed when the percentage of identification was >90%. As for MALDI-TOF analysis, when a probability score between 60% and 100% represents a high discrimination value and a reliable identification, MALDI-TOF MS identification was considered final. Discrepant results were regarded as follows: (I) a probability score that is >60% is found in a low discrimination identification that consists of a list of two to four choices for an identification match; (II) scores of <60% were considered to have no valid identification; and (III) a report of no identification is produced when either no match is found for the composite spectra, or not enough spectral peaks were obtained in the analysis. In the case of discrepant results or no identification with one or both methods, 16S rDNA or ITS sequencing resolved final identification.
Sequence data
Isolates that yielded discrepant results between routine phenotypic identification and MALDI-TOF MS identification were subjected to partial 16S rDNA or ITS gene sequencing by an outside reference laboratory. DNA was extracted with the MagNAPure LC DNA isolation kit II (Roche Molecular Biochemicals, Mannheim, Germany) according to the manufacturer’s instructions. PCR amplification of gene was performed using the primers for 16S rDNA (F-AGAGTTTGATCCTGGCTCAG and R-TACGGCTACCTTGTTACGACTT) or the primers for ITS (F-TCCGTAGGTGAACCTGCGG and R-TCCTCCGCTTATTGATATGC). Amplicons were purified and double-strand sequenced using the primers for 16S rDNA or ITS. Fragments were analyzed using an automatic DNA sequencer (ABI Prism 3730 XL genetic analyzer; Applied Biosystems, Foster City, CA, USA) and queried against NCBI/GenBank databases. A per cent similarity of ≥99% between the unknown sequence and the closest matching sequence from the GenBank database was used as the criterion to classify an isolate to the species level.
Calculation of global assessment indices
For the MALDI-TOF based identification method, positive predictive values to the genus level and to the species level, considering isolates with correct identifications to the genus level true positives and isolates with correct identifications to the species level true positives, respectively, were calculated. Misidentified isolates were considered false positives. Negative predictive value, considering isolates with an absence of identification and belonging to species not included in the database true negatives and isolates with an absence of identifications and belonging to species included in the database false negatives, was calculated.
Results
Global identification performances
During the study period, 1,181 isolates were analyzed by the Vitek® MS system and the conventional Vitek2-compact system in parallel. Implementation of DNA-based identification methods to manage discrepancies or to obtain a more accurate fine identification (to the species or subspecies level) was performed for 9 (0.8%) isolates. Fine identification proposed by the Vitek® MS as a single choice, whatever the confidence value, or included in a multiple-choice result were considered overall correct identifications. Of 1,181 isolates encompassing 80 species and 39 genera, 1,175 (99.5%) isolates were correctly identified by MALDI-TOF MS as defined previously (Table 1). No IDs and discordant results (mis-IDs) were obtained for 0.4% and 0.1% of the isolates, respectively.
Correct MALDI-TOF mass spectrometry identifications
Among the 1,181 isolates, 1,130 (95.7%) isolates had correct species identification using Vitek® MS. Results of MALDI-TOF MS identifications for Enterobacteriaceae, nonfermentative Gram-negative rods, a group of miscellaneous bacteria, Gram-positive cocci, anaerobes and yeast are depicted separately in Table 1. A correct identification to the genus level only, that is, the correct species ID was included in a multiple-choice result of species from the same genus, was obtained for 3.6% (n=42) of the isolates including species complexes such as Enterobacter cloacae/asburiae (n=36), Pantoea agglomerans/dispersa (n=1), Salmonella spp (n=1), Acinetobacter junii/johnsonii (n=1), and Aeromonas hydrophila/caviae (n=3). Low discrimination results above the genus level, that is, with the correct ID proposed among species of different genera, were obtained for 0.3% (n=3) of the isolates (Table 1), some of which also seem to be recurrent, like the Moraxella osloensis/Alcaligenes faecalis low discrimination result for Moraxella osloensis isolate. An identical and high confidence value was mostly obtained for each proposed species in the case of a low discrimination result to the species level or above the genus level. In the few cases in which a confidence value difference occurred, it argued either for or against the correct species ID.
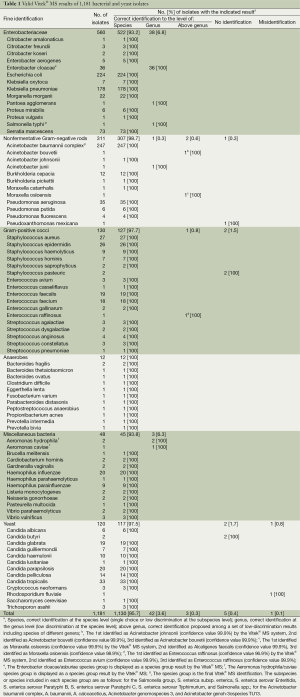
Full table
Lack of identification and erroneous MALDI-TOF mass spectrometry identification
The Vitek® MS system gave an absence of identification for 5 (0.4%) isolates that were tested again using one deposit for informative purposes (Table 2). These isolates included one Pseudoxanthomonas mexicana isolate, two Staphylococcus pasteuric isolates, and two Candida butyri isolates, for which the system gave the same “no identification” answer after reading a second deposit. These isolates among three tested in the study again gave a “no identification” result despite the additional retest. An additional one Rhodosporidium fluviale isolate (0.1%) was erroneously identified as Rhodotorula glutinis by MALDI-TOF mass spectrometry even though a confidence value of 99.9% was obtained (Table 2).
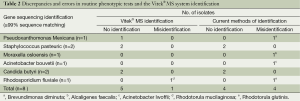
Full table
Phenotype erroneous identifications
The current methods of identification failed for four isolates (0.3%), which were two Staphylococcus strains and two strains of yeast (Table 2). Phenotypic identification was erroneous for four isolates (0.3%). One isolate phenotypically identified as Brevundimonas diminuta was not identified by MALDI-TOF mass spectrometry and was confirmed to be Pseudoxanthomonas mexicana by 16S rDNA gene sequencing. One isolate phenotypically identified as Alcaligenes faecalis was identified as Moraxella osloensis by MALDI-TOF mass spectrometry and as Moraxella osloensis by 16S rDNA gene sequencing. One isolate phenotypically identified as Acinetobacter lwoffii was identified as Acinetobacte bouvetii by MALDI-TOF mass spectrometry and as Acinetobacter bouvetii by 16S rDNA gene sequencing. One isolate phenotypically identified as Rhodotorula glutinis isolate was identified as Rhodotorula mucilaginosa by MALDI-TOF mass spectrometry and was confirmed to be Rhodosporidium fluviale by ITS gene sequencing.
Global assessment indices
According to the criteria detailed in the Materials and methods section, all of the positive predictive values to the genus level and to the species level of the Vitek® MS system were 99.7, and the negative predictive value was 83.3.
Discussion
Culturing, isolating, and then identifying microorganisms remains the gold standard procedure in etiological diagnosis of infectious diseases. However, for many fastidious pathogens, including Mycobacterium tuberculosis and various fungi species, the growth cycle can be long and tenuous, and the costs associated with longer turn-around-times to phenotypic identification for these organisms are quite high. Matrix assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS) based proteomics has quickly developed in recent years in the area of microbiology. The basic principle of MALDI-TOF MS technology is the use of a matrix solution which co-crystallization of sample and matrix substrate following solvent evaporation. The matrix-sample formation will absorb the energy when fired upon by a laser, transferring the ionic charge from matrix to sample. When the charged samples enter the vacuum tube and accelerating electric field of the system, the charged sample fragments will be separated based on their mass-to-charge ratio, and the flight detector analyzes this separation based on mass and charge and generates what is known as mass spectra. Using software and algorithmic analysis, the mass spectra for the sample will be compared against the mass spectra for known species contained in the system database.
The VITEK®-MS v2.0 MALDI-TOF system has been implemented in our laboratory for efficient, cost-effective, rapid, and routine identification of bacterial and yeast isolates (14,17,18). The results of our prospective analysis of 1,181 clinical isolates revealed exceptional performance of the VITEK®-MS v2.0 MALDI-TOF system in comparison to conventional identification techniques. Overall, the performance of this system was highly accurate (95.7% correct to species-level identification), where only 0.5% of the total tested isolates were not identified or misidentified.
Until now, most studies have reported on the proof-of-concept of MALDI-TOF MS for specific microorganisms (6,9,12,13,19). The majority of these studies included strains from reference and culture collections. Recently, a study by Seng et al. concluded that MALDI-TOF MS can replace conventional systems for identification of bacteria in a conventional laboratory (20). In our study, the performance of MALDI-TOF MS was specified in detail for different groups of microorganisms. In accordance with the results of Seng et al., >95% of our clinical isolates could be identified to species levels by MALDI-TOF MS. Enterobacteriaceae and Nonfermentative Gram-negative rods, both clinically relevant pathogens accounting for a large majority of aerobic Gram-negative rods in a conventional medical microbial laboratory, were accurately identified to the species level (>93%), even for very closely related species.
In the present study, the VITEK®-MS system was not able to distinguish Enterobacter cloacae isolates from Enterobacter asburiae, while the conventional systems correctly identified all isolates to the species level. Similarly, the VITEK®-MS system could not resolve identification for Aeromonas hydrophila/caviae. These findings are consistent with other reports in the literature (21). This may be because species show similar pathogenicities and antibiotics susceptibility patterns (for example, Enterobacter cloacae/asburiae), thus requiring some biochemical tests (for example, exercise test for Enterobacter cloacae/asburiae, VP test for Aeromonas hydrophila/caviae) to distinguish. These results clearly demonstrate that updating the database is essential for bacterial identification and that there are improvements to be made on the current database.
An important advantage of MALDI-TOF MS is rapid identification gram-positive cocci, including staphylococci, enterococci and streptococci. Coagulase negative staphylococcus (CoNS) are the most common blood isolates in culture, accounting for about 45% of the isolated bacteria in blood culture. However, about 60-80% CoNS isolated from blood culture are suspected to be contaminated bacteria. With the increase of invasive surgical operations, 27-38% catheter-related bloodstream infection is estimated to be caused by CoNS. So identification to the exact species level may be very useful as some CoNS can contaminate cultures from true infections by staphylococcus species. Most studies report a number of viridans streptococci and pneumococci were misidentified by MALDI-TOF MS. Seng et al. found that nearly 50% of S. pneumoniae isolates were misidentified as Streptococcus parasanguinis because the database included only three S. pneumoniae and two S. parasanguinis reference spectra (20). Therefore, the database also needs improvement, with more spectra of well-identified streptococcal species. However, in our study the performance of MALDI-TOF MS was specified in the identification of viridans streptococci and pneumococci. This may be related to the number of identification bacteria associated with the existing database.
MALDI-TOF MS performed well for identification of yeasts in our study, with correct identification of 97.5% of 120 isolates encompassing 13 different species without laborious sample preparation procedures. Over the past decade the significance of infections by yeasts has increased, especially those caused by germ tube negative yeasts. Given the variable susceptibility of different species of yeasts to antifungal agents, the rapid and correct identification is of clinical importance. Furthermore, the identification of yeast isolates to the species level makes it possible to study the epidemiology of colonization and infection and the transmission of infections in hospitals. Conventional identification methods, however, are laborious and time-consuming. Additionally, high-resolution DNA-based molecular techniques, such as 16S or 18S rRNA or ITS DNA sequencing and real-time PCR assays, are expensive and also time-consuming. Lohmann et al. studied 312 clinical isolates and concluded that MALDI-TOF MS is a rapid and reliable tool for the identification of yeasts and yeast-like fungi, with low expenditure of consumables, easy interpretation of results, and a fast turnaround time (16). Misidentifications in our study and the Lohmann study were attributed to the use of an incomplete database.
In the study presented here, five isolates were insufficiently identified because of missing reference spectra in the VITEK®-MS database: Pseudoxanthomonas Mexicana, Staphylococcus pasteuric (n=2) and Candida butyri (n=2) were declared “unknown spectrum”, and Rhodosporidium fluviale was misidentified as Rhodotorula mucilaginosa. This misidentification may be due to a technical error during sample preparation on the target slide.
The strengths of our study are the implementation of MALDI-TOF MS in a routine setting, the comparison of MALDI-TOF MS with conventional identification systems on clinical isolates, the use of 16S rDNA and ITS sequencing for analysis of discrepancies, and the inclusion of yeasts in addition to bacteria. The main limitation of this study is the lack of inclusion of sufficient Gram-positive aerobic rods and enteropathogens. During the study period, aerobic Gram-positive rods and enteropathogens were isolated sporadically (n=2 and n=8 isolates, respectively). Conventional identification methods for aerobic Gram-positive rods are cumbersome and time consuming. Using MALDI-TOF MS for identifying aerobic Gram-positive rods would certainly increase the number of species identifications since it can be applied directly from bacterial colonies on the primary culture plates (11,22). Moreover, MALDI-TOF MS technology is a powerful tool that can be used in routine laboratories for the diagnosis of enteric diseases. It is particularly useful for the rapid discrimination of Normal flora from potential pathogens that are isolated from stool samples. For pathogen identification itself, the limitations of MALDI-TOF MS must be considered. Initially, the identification of Shigella or E. coli will still require additional tests according to the nature of the sample (20,23). Secondly, biochemical and serological tests will still be required to accurately identify Salmonella species (23). Additional studies should be conducted in order to evaluate the ability of the Vitek MS database to differentiate S. typhi from other Salmonella serotypes. This indeed is of major interest from both the clinical management and public health perspectives.
Our results suggest that the major factors that may influence the quality of MALDI-TOF MS identifications are the purity of the strain, the amount of biological material smeared on the target plate and the experience of the technologist. Without an intensive training background of the technicians, the technical ownership of the Vitek® MS system is straightforward and fast, as previously mentioned (24). However, the technician must remain vigilant in routine practice during sample preparation because of reduced interspot distances (especially for spots near the E. coli calibrant spot) that can mix two bacterial deposits, particularly during the matrix application step, as happened during the training period. In addition, it is extremely important to cultivate conditions and colony solvent treatment. Need to strictly according to the manufacturer’s operating standards, in order to ensure the consistency of the identification results.
Rapid and reliable identification of bacteria and fungi is paramount for effective therapy. The VITEK®-MS is an accurate system for identifying clinically relevant bacteria and yeasts with only one deposit of crude bacteria and yeasts, and without any extraction step required. Implementation of this technology in the clinical microbiology laboratory will lead to decreased turnaround times for identification, having a large impact on clinical outcome and dramatically reducing healthcare costs. MALDI-TOF MS can also be used in addition to traditional methods, such as colony morphology and Gram stain tests, for organisms that are difficult to identify. The introduction of MALDI-TOF MS into the clinical microbiology laboratory represents a significant shift in the diagnosis of bacterial and yeast infections, and ultimately enhances patient care.
Conclusions
In summary, MALDI-TOF MS-based identification provides cheaper and faster bacterial and yeast species identification than conventional phenotypic identification methods, with equal or better accuracy. Our results demonstrate that the VITEK® MS system is a rapid and reliable technique, and has the potential to replace conventional phenotypic identification for most bacterial and yeast strains routinely isolated in clinical microbiology laboratories. However, spectral databases should be regularly updated by suppliers to improve identification rates.
Acknowledgements
This work was supported by Medical Science and Technology Research Projects of PLA Foundation (CWS129811298) and Medical Science and Technology Innovation Projects of Nanjing Military Area Foundation (10MA101).
Disclosure: The authors declare no conflict of interest.
References
- Carroll KC, Weinstein MP. Manual and automated systems for detection and identification of microorganisms. In: Murray PR, Baron EJ, Jorgensen JH, et al. eds. Manual of clinical microbiology. 9th ed. Washington, DC: American Society for Microbiology Press 2007:192-217.
- Klouche M, Schröder U. Rapid methods for diagnosis of bloodstream infections. Clin Chem Lab Med 2008;46:888-908. [PubMed]
- Krishnamurthy T, Ross PL, Rajamani U. Detection of pathogenic and non-pathogenic bacteria by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 1996;10:883-8. [PubMed]
- Demirev PA, Ho YP, Ryzhov V, et al. Microorganism identification by mass spectrometry and protein database searches. Anal Chem 1999;71:2732-8. [PubMed]
- Bernardo K, Pakulat N, Macht M, et al. Identification and discrimination of Staphylococcus aureus strains using matrix-assisted laser desorption/ionization-time of flight mass spectrometry. Proteomics 2002;2:747-53. [PubMed]
- Moura H, Woolfitt AR, Carvalho MG, et al. MALDI-TOF mass spectrometry as a tool for differentiation of invasive and noninvasive Streptococcus pyogenes isolates. FEMS Immunol Med Microbiol 2008;53:333-42. [PubMed]
- Conway GC, Smole SC, Sarracino DA, et al. Phyloproteomics: species identification of Enterobacteriaceae using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Mol Microbiol Biotechnol 2001;3:103-12. [PubMed]
- Mellmann A, Cloud J, Maier T, et al. Evaluation of matrix-assisted laser desorption ionization-time-of-flight mass spectrometry in comparison to 16S rRNA gene sequencing for species identification of nonfermenting bacteria. J Clin Microbiol 2008;46:1946-54. [PubMed]
- Miñán A, Bosch A, Lasch P, et al. Rapid identification of Burkholderia cepacia complex species including strains of the novel Taxon K, recovered from cystic fibrosis patients by intact cell MALDI-ToF mass spectrometry. Analyst 2009;134:1138-48. [PubMed]
- Schaumann R, Knoop N, Genzel GH, et al. Discrimination of Enterobacteriaceae and Non-fermenting Gram Negative Bacilli by MALDI-TOF Mass Spectrometry. Open Microbiol J 2013;7:118-22. [PubMed]
- Lefmann M, Honisch C, Böcker S, et al. Novel mass spectrometry-based tool for genotypic identification of mycobacteria. J Clin Microbiol 2004;42:339-46. [PubMed]
- Grosse-Herrenthey A, Maier T, Gessler F, et al. Challenging the problem of clostridial identification with matrix-assisted laser desorption and ionization-time-of-flight mass spectrometry (MALDI-TOF MS). Anaerobe 2008;14:242-9. [PubMed]
- Nagy E, Maier T, Urban E, et al. Species identification of clinical isolates of Bacteroides by matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry. Clin Microbiol Infect 2009;15:796-802. [PubMed]
- Garner O, Mochon A, Branda J, et al. Multi-centre evaluation of mass spectrometric identification of anaerobic bacteria using the VITEK® MS system. Clin Microbiol Infect 2014;20:335-9. [PubMed]
- Lacroix C, Gicquel A, Sendid B, et al. Evaluation of two matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) systems for the identification of Candida species. Clin Microbiol Infect 2014;20:153-8. [PubMed]
- Lohmann C, Sabou M, Moussaoui W, et al. Comparison between the Biflex III-Biotyper and the Axima-SARAMIS systems for yeast identification by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 2013;51:1231-6. [PubMed]
- Iriart X, Lavergne RA, Fillaux J, et al. Routine identification of medical fungi by the new Vitek MS matrix-assisted laser desorption ionization-time of flight system with a new time-effective strategy. J Clin Microbiol 2012;50:2107-10. [PubMed]
- Fothergill A, Kasinathan V, Hyman J, et al. Rapid identification of bacteria and yeasts from positive-blood-culture bottles by using a lysis-filtration method and matrix-assisted laser desorption ionization-time of flight mass spectrum analysis with the SARAMIS database. J Clin Microbiol 2013;51:805-9. [PubMed]
- Yang YC, Yu H, Xiao DW, et al. Rapid identification of Staphylococcus aureus by surface enhanced laser desorption and ionization time of flight mass spectrometry. J Microbiol Methods 2009;77:202-6. [PubMed]
- Seng P, Drancourt M, Gouriet F, et al. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis 2009;49:543-51. [PubMed]
- Dubois D, Grare M, Prere MF, et al. Performances of the Vitek MS matrix-assisted laser desorption ionization-time of flight mass spectrometry system for rapid identification of bacteria in routine clinical microbiology. J Clin Microbiol 2012;50:2568-76. [PubMed]
- Ryzhov V, Hathout Y, Fenselau C. Rapid characterization of spores of Bacillus cereus group bacteria by matrix-assisted laser desorption-ionization time-of-flight mass spectrometry. Appl Environ Microbiol 2000;66:3828-34. [PubMed]
- Neville SA, Lecordier A, Ziochos H, et al. Utility of matrix-assisted laser desorption ionization-time of flight mass spectrometry following introduction for routine laboratory bacterial identification. J Clin Microbiol 2011;49:2980-4. [PubMed]
- Bizzini A, Durussel C, Bille J, et al. Performance of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of bacterial strains routinely isolated in a clinical microbiology laboratory. J Clin Microbiol 2010;48:1549-54. [PubMed]


