Emergence of linezolid resistance in a clinical Staphylococcus capitis isolate from Jiangsu Province of China in 2012
Introduction
Linezolid (LZD) is an oxazolidinone antibacterial agent approved by FDA of America in the year of 2000 for the treatment of infection with gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (1), glycopeptide-intermediate S. aureus and vancomycin-resistant Enterococci. It exerts its antibacterial activity by acting on the early stage of the protein synthesis process; it binds to the ribosomal 23S portion of the 50S subunt of target bacteria and thereby inhibits the formation of the 70S initiation complex (2). LZD resistance occurs by mutations in the LZD 23S rRNA binding site, the ribosomal proteins L3 and/or L4 of the peptide translocation centre of the ribosome or by acquisition of a plasmid-borne ribosomal methyltransferase gene, cfr (3,4). In 2001, the first LZD-resistant S. aureus was reported in a US patient who had received a 1 month LZD treatment for dialysis-associated peritonitis (5). Since then, cases of LZD-resistant Staphylococcus have been reported worldwide in America (5-7), Europe (8-10) and Asia (11,12). In China, LZD was approved into clinical use in 2007 and several clinical cases of LZD-resistant Staphylococcus capitis have emerged in Zhejiang Province and the city of Beijing (13,14), but this is the first report, to our knowledge, of LZD-resistant Staphylococcus capitis in Jiangsu of China. The strain was isolated from blood sample of a patient with severe pulmonary infection. It showed an apparent resistance to LZD and was proved to have C2190T and C2561Y point mutations in the 23S rRNA and carry the cfr gene.
Case report
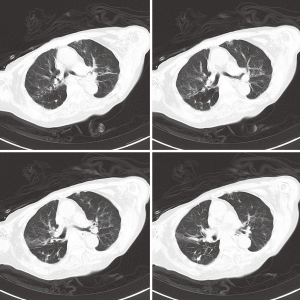
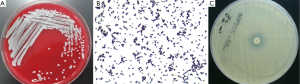
In August 2012, a 92-year old female who suffered recurrent pulmonary infections in recent two years was admitted to the geriatrics department because of the onset of cough and sputum for two days. The following CT scan showed that she got pneumonia (Figure 1). She was started with ceftazidime on an empirical treatment with moxifloxacin but showed no response to the treatment and developed a fever (39.0 °C) two days after admission. The subsequent bacterial cultures showed the coexistence of Pseudomonas aeruginosa, Acinetobacter baumannii and Aspergillus in her sputum sample and Enterococcus avium in the urine sample. Then we used diflucan to resist the fungal and ceftazidime, cefoperazone/sulbactam, imipenem, fosfomycin and tigecycline successively to combat the bacterial infection. To resist the gram-positive cocci, LZD was used on the 20th day after the patient’s admission with a dosage of 1,200 mg/d, and then the drug was discontinued 11 days later. Though the count of white blood cells (WBC) came to a transient decrease (drop from 19.5×109/L to 11.8×109/L) after the use of the antibiotics above, the patient eventually died of a sudden onset of ventricular tachycardia on the 35th day after admission. Patient in this case had long-term hospitalization history because of her recurrent pulmonary infection and had accepted lots antibiotics treatments. During her last hospitalization, the patient accepted airway intubation because of typeII respiratory failure and femoral vein catheterization to conduct hemodialysis for her renal failure. Because of her continued fever (varied between 37.0 and 39.0 °C), we conducted bilateral double bottles for blood cultures repeatedly to confirm the existence of bacteremia. We performed blood culture for three times, the first two (sampling on the 15th and 21th days after admission, respectively) both showed negative results whereas the last one (sampling on the 28th day after admission) showed a positive result. A strain of Staphylococcus capitis was isolated in last blood culture by using Viteck 2 compact (Figure 2A,B), and the strain showed resistance to LZD using the K-B method (Figure 2C) and also show high resistance with a MIC of 64 μg/mL using the broth dilution method. The Staphylococcus capitis also showed resistance to penicillin, pipercillin/tazobactam, cefepime, amikacin, levofloxacin, clindamycin while it was susceptible to cotrimoxazole, vancomycin and teicoplanin. Antimicrobial sensitivity test was conducted by the K-B disc diffusion method in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines (15). Domain V region of the 23S rRNA gene spanning 2001 to 2597bp (E. coli numbering) was amplified. Oligonucleotide premiers 5'-TGG GCA CTG TCT CAA CGA-3' and 5'-GGA TAG GGA CCG AAC TGT CTC-3' were used to amplify a 596bp fragment. Polymerase chain reaction (PCR) conditions were 30 cycles consisting of 94 °C for 1 min, for 30 s at 50 °C sec, and 72 °C for 1 min. The PCR fragments (596 bp) were purified and sequenced and the strain was found to have C2190T and C2561Y point mutations in the 23S rRNA. We also confirmed the existence of cfr gene in the Staphylococcus capitis using the method of PCR which was described in previous reports (16,17).
Disccusion
LZD is used for the treatment of infection with gram-positive bacteria, and had curative effect constantly since it was approved in 2000. Data from the USA and global surveillance studies report of LZD resistance were Staphylococcus aureus and 2% of coagulase-negative Staphylococcus (10,18-21).
A systematic review has shown that the mean time of LZD therapy reported prior to isolation of LZD-resistance coagulase-negative was 20 months, significant longer than case of LZD-resistance Staphylococcus (11 days) (22). In our case, the LZD resistance Staphylococcus capitis was isolated from the patient’s blood sample only eight days after the use of LZD, which suggested that short-term use of LZD may also induce the LZD-resistance coagulase-negative Staphylococcus. The cfr gene which is capable of transmitting horizontally between species is also an important factor leading to the LZD resistance. So we suggest that effective infection control measures should be enhanced to prevent the spread of multi-drug resistant strains.
The LZD resistance S. capitis in this case was isolated from a blood sample, which was documented in the study of Gu B et al. (22) that the most common samples cultured the LZD resistance coagulase-negative Staphylococcus. So when patients undergo conditions similar to this case: suffering a symptom of high fever, receiving invasive operations such as airway intubation, deep vein catheterization and so on, it is important for the clinicians to conduct blood culture to confirm whether there is a Staphylococcus induced blood stream infection. Timely detection of LZD resistance Staphylococcus is of great significance in the rational use of antibiotics and avoiding the emergence of multi-drug resistance bacteria.
Regarding to the mechanism of LZD resistance, the main explanation is mutations in the domain V region of the 23S rRNA gene. While the C2190T mutation was reported in several isolates of S. homin (23), this is the first report, to our knowledge, of C2190T and C2561Y mutations in Staphylococcus capitis. The isolate was also PCR-positive for the cfr, a gene located on a transferable element, which indicated a potential to disseminate horizontally among Gram-positive pathogenic strains.
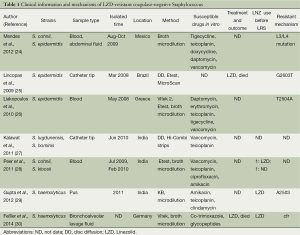
This article systematically reviews the published literature for case reports of LZD-resistant coagulase-negative Staphylococcus (LRCoNS) (Table 1). In all reported cases, strains including S. cohnii, S. epidermidis, S. lugdunensis, S. hominis and S. kloosii were isolated from aseptic sample, which included blood, pus, bronchoalveolar lavage fluid. Nevertheless blood sample was the vast majority. LRCoNS were reported worldwide, including North America, South America, European and Asia. All these strains reported were conducted the susceptibility test by broth microdilution. The mechanisms for LZD resistance were L3/L4 mutation, G2603T, T2504A, A2503 mutations in the 23S rRNA and the presence of a transmissible cfr ribosomal methyltransferase. The outcome of the LRCoNS infected patients is obscure, however, two cases mentioned the patients were died in the end, which had the same outcome in our report.
Full table
In conclusion, though the LZD-resistant Staphylococcus is still sporadic now, the prolonged hospital stays, frequent interventions and abuse of antibiotics may accelerate the dissemination of LZD resistance Staphylococcus. Judicious use of LZD and surveillance of resistance in staphylococci are necessary to preserve the therapeutic efficacy of this important antimicrobial.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Chien JW, Kucia ML, Salata RA. Use of LZD, an oxazolidinone, in the treatment of multidrug-resistant gram-positive bacterial infections. Clin Infect Dis 2000;30:146-51. [PubMed]
- Shinabarger D. Mechanism of action of the oxazolidinone antibacterial agents. Expert Opin Investig Drugs 1999;8:1195-202. [PubMed]
- Zhu W, Tenover FC, Limor J, et al. Use of pyrosequencing to identify point mutations in domain V of 23S rRNA genes of LZD-resistant Staphylococcus aureus and Staphylococcus epidermidis. Eur J Clin Microbiol Infect Dis 2007;26:161-5. [PubMed]
- Meka VG, Pillai SK, Sakoulas G, et al. LZD resistance in sequential Staphylococcus aureus isolates associated with a T2500A mutation in the 23S rRNA gene and loss of a single copy of rRNA. J Infect Dis 2004;190:311-7. [PubMed]
- Tsiodras S, Gold HS, Sakoulas G, et al. LZD resistance in a clinical isolate of Staphylococcus aureus. Lancet 2001;358:207-8. [PubMed]
- Gales AC, Sader HS, Andrade SS, et al. Emergence of LZD-resistant Staphylococcus aureus during treatment of pulmonary infection in a patient with cystic fibrosis. Int J Antimicrob Agents 2006;27:300-2. [PubMed]
- Toh SM, Xiong L, Arias CA, et al. Acquisition of a natural resistance gene renders a clinical strain of methicillin-resistant Staphylococcus aureus resistant to the synthetic antibiotic LZD. Mol Microbiol 2007;64:1506-14. [PubMed]
- Sánchez García M, De la Torre MA, Morales G, et al. Clinical outbreak of LZD-resistant Staphylococcus aureus in an intensive care unit. JAMA 2010;303:2260-4. [PubMed]
- Hill RL, Kearns AM, Nash J, et al. LZD-resistant ST36 methicillin-resistant Staphylococcus aureus associated with prolonged LZD treatment in two paediatric cystic fibrosis patients. J Antimicrob Chemother 2010;65:442-5. [PubMed]
- Ross JE, Farrell DJ, Mendes RE, et al. Eight-year (2002-2009) summary of the LZD (Zyvox® Annual Appraisal of Potency and Spectrum; ZAAPS) program in European countries. J Chemother 2011;23:71-6. [PubMed]
- Ikeda-Dantsuji Y, Hanaki H, Sakai F, et al. LZD-resistant Staphylococcus aureus isolated from 2006 through 2008 at six hospitals in Japan. J Infect Chemother 2011;17:45-51. [PubMed]
- Yoshida K, Shoji H, Hanaki H, et al. LZD-resistant methicillin-resistant Staphylococcus aureus isolated after long-term, repeated use of LZD. J Infect Chemother 2009;15:417-9. [PubMed]
- Cai JC, Hu YY, Zhang R, et al. LZD-resistant clinical isolates of meticillin-resistant coagulase-negative staphylococci and Enterococcus faecium from China. J Med Microbiol 2012;61:1568-73. [PubMed]
- Chen H, Wu W, Ni M, et al. LZD-resistant clinical isolates of enterococci and Staphylococcus cohnii from a multicentre study in China: molecular epidemiology and resistance mechanisms. Int J Antimicrob Agents 2013;42:317-21. [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement 2012;32:M100-S22.
- Morales G, Picazo JJ, Baos E, et al. Resistance to LZD is mediated by the cfr gene in the first report of an outbreak of LZD-resistant Staphylococcus aureus. Clin Infect Dis 2010;50:821-5. [PubMed]
- Kehrenberg C, Schwarz S. Distribution of florfenicol resistance genes fexA and cfr among chloramphenicol-resistant Staphylococcus isolates. Antimicrob Agents Chemother 2006;50:1156-63. [PubMed]
- Jones RN, Kohno S, Ono Y, et al. ZAAPS International Surveillance Program (2007) for LZD resistance: results from 5591 Gram-positive clinical isolates in 23 countries. Diagn Microbiol Infect Dis 2009;64:191-201. [PubMed]
- Jones RN, Ross JE, Castanheira M, et al. United States resistance surveillance results for LZD (LEADER Program for 2007). Diagn Microbiol Infect Dis 2008;62:416-26. [PubMed]
- Sader HS, Jones RN. Antimicrobial susceptibility of Gram-positive bacteria isolated from US medical centers: results of the Daptomycin Surveillance Program (2007-2008). Diagn Microbiol Infect Dis 2009;65:158-62. [PubMed]
- Farrell DJ, Mendes RE, Ross JE, et al. LZD surveillance program results for 2008 (LEADER Program for 2008). Diagn Microbiol Infect Dis 2009;65:392-403. [PubMed]
- Gu B, Kelesidis T, Tsiodras S, et al. The emerging problem of LZD-resistant Staphylococcus. J Antimicrob Chemother 2013;68:4-11. [PubMed]
- Sorlozano A, Gutierrez J, Martinez T, et al. Detection of new mutations conferring resistance to LZD in glycopeptide-intermediate susceptibility Staphylococcus hominis subspecies hominis circulating in an intensive care unit. Eur J Clin Microbiol Infect Dis 2010;29:73-80. [PubMed]
- Mendes RE, Deshpande LM, Costello AJ, et al. Molecular epidemiology of Staphylococcus epidermidis clinical isolates from U.S. hospitals. Antimicrob Agents Chemother 2012;56:4656-61. [PubMed]
- Lincopan N, de Almeida LM, Elmor de Araújo MR, et al. LZD resistance in Staphylococcus epidermidis associated with a G2603T mutation in the 23S rRNA gene. Int J Antimicrob Agents 2009;34:281-2. [PubMed]
- Liakopoulos A, Spiliopoulou I, Damani A, et al. Dissemination of two international LZD-resistant Staphylococcus epidermidis clones in Greek hospitals. J Antimicrob Chemother 2010;65:1070-1. [PubMed]
- Kalawat U, Sharma KK, Reddy S. LZD-resistant Staphylococcus spp. at a tertiary care hospital of Andhra Pradesh. Indian J Med Microbiol 2011;29:314-5. [PubMed]
- Peer MA, Nasir RA, Kakru DK, et al. Sepsis due to LZD resistant Staphylococcus cohnii and Staphylococcus kloosii: first reports of LZD resistance in coagulase negative staphylococci from India. Indian J Med Microbiol 2011;29:60-2. [PubMed]
- Gupta V, Garg S, Jain R, et al. LZD resistant Staphylococcus haemolyticus: first case report from India. Asian Pac J Trop Med 2012;5:837-8. [PubMed]
- Feßler AT, Calvo N, Gutiérrez N, et al. Cfr-mediated LZD resistance in methicillin-resistant Staphylococcus aureus and Staphylococcus haemolyticus associated with clinical infections in humans: two case reports. J Antimicrob Chemother 2014;69:268-70. [PubMed]