Atypical pulmonary alveolar proteinosis presenting as a mixed nodular ground-glass opacity with focal mucinosis mimicking lung cancer
Introduction
There are various guidelines for the management of a single pulmonary nodule. Although liquid biopsy alone may be able to diagnose lung cancer safely and easily in the future, clinical approaches based on radiological (CT with or without PET) appearance and cancer risk factors are currently common in guidelines. In addition, there are several prediction models that estimate the clinical probability of malignancy in a pulmonary nodule based on radiological appearance and cancer risk factors (1-3). In Asia, the American College of Chest Physicians (ACCP) guidelines for Asia are used for the management of a single pulmonary nodule (4). However, there is no useful prediction model validated in Asian populations, and the high prevalence of granulomatous and other infectious diseases, which may present as pulmonary nodules, needs to be considered in Asia (4). Furthermore, in clinical practice, we occasionally encounter cancer-negative specimens for which a histologically definitive diagnosis is difficult, or diagnostic outcomes are surprising.
Pulmonary alveolar proteinosis (PAP) is a relatively rare syndrome characterized by an unreasonable accumulation of eosinophilic and periodic acid-Schiff (PAS) staining-positive material within the alveoli generally due to insufficient surfactant catabolism by macrophages. The material is usually fine granular, but is occasionally condensed into large globules (5,6). The pathophysiologies of PAP can be classified into three groups: congenital surfactant proteins (SPs) or granulocyte macrophage-colony stimulating factor (GM-CSF) receptor gene mutations; secondary functional disorder or decreased numbers of alveolar macrophages due to various conditions such as haematologic malignancy and toxic inhalation; and idiopathic (the majority of this group have been found to be anti-GM-CSF antibody-positive and classified as autoimmune PAP, which accounts for more than 90% of all PAP cases) (7). The usual chest radiographic finding of PAP is diffuse airspace opacity showing a perihilar and basal distribution without pleural effusion. The high-resolution (HR) CT image of ground-glass opacity (GGO) combined with inter- and intralobular septal thickening, the so called ‘‘crazy-paving’’ pattern, is a representative finding of PAP (8). In advanced PAP, confirmation of a typical crazy-paving appearance combined with milky PAS-positive bronchoalveolar lavage suggests a PAP diagnosis. However, the pattern is not present in approximately 1/5 of cases, and up to 1/3 of patients may be asymptomatic (9). Therefore, the pathophysiology of early stage PAP is not fully elucidated. In addition, localized PAP, which manifests no clinical symptoms, is extremely rare and, to the best of our knowledge, such case has not been reported in non-Asian patient (10-13). In this manuscript, we report an atypical anti-GM-CSF antibody-negative PAP case presenting as a mixed nodular GGO with focal mucinosis, which was not expected in a standard single pulmonary nodule diagnostic pathway.
Case presentation
A 71-year-old male visited a nearby hospital due to persistent dry cough following upper respiratory tract infection, and a 12-mm nodular GGO was detected in his right lower lobe by chest CT performed on suspicion of interstitial pneumonia (Figure 1A). Although a CT scan was scheduled after 2 months, the patient stopped visiting the hospital as the symptom disappeared after a short time. However, when the patient visited the hospital again 9 months later, concerned that he had not received follow-up observation, CT revealed an increase in density of the nodular GGO (Figure 1B). The patient was then referred to our hospital on clinical suspicion of lung cancer.
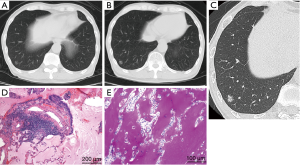
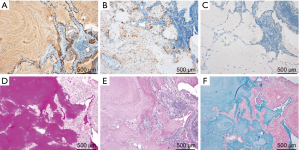
The patient had no clinical history of COPD, pulmonary infections, extrathoracic cancers, haematologic malignancies, autoimmune diseases, dust or toxic inhalation, but he had smoked 5 cigarettes a day for 51 years (12.75 pack-years) up to the hospital visit. The clinical probability of malignancy based on a prediction model described in the ACCP guidelines for Asia on the evaluation of pulmonary nodules was 15% (moderate) (3,4). The prediction model reflects age, smoking status, history of cancers and diameter, spiculation, and location of the nodule. Laboratory data were as follows: white blood cell count, 6,170/µL (neutrophils, 60.9%; lymphocytes, 33.2%; monocytes, 4.1%; basophils, 1.0%; eosinophils, 0.8%; abnormal cells, 0.0%); hemoglobin, 13.9 g/dL; platelet count, 18.9×104/all; AST, 13 IU/L; ALT, 10 IU/L; γGTP, 35 IU/L; LDH, 117 IU/L; Na, 145 mEq/L; K, 4.0 mEq/L; Cl,109 mEq/L; creatinine, 0.83mg/dl; C-reactive protein, 0.03 mg/dL. HRCT showed a mixed (or part-solid) nodular GGO with a slightly dilated air bronchogram, suggesting a well differentiated adenocarcinoma (Figure 1C) (14). Interstitial pneumonia was not observed. We originally planned to carry out a CT-guided transthoracic needle biopsy (CT-TNB). However, the patient preferred to undergo video-assisted thoracoscopic partial resection of the right lower lobe to get a definite diagnosis. After preoperative evaluation by the thoracic surgeon, partial resection was completed without complications. Histologically, no neoplastic cells were present in the resected specimen and the alveolar spaces were filled with eosinophilic and PAS staining-positive material throughout the entire volume of the nodular GGO indicating PAP (Figure 1D,E). Eosinophilic large globules were not contained (Figure 1D,E). Epithelial hyperplasia of bronchiole and fibrosis under the epithelium were observed in the nodular lesion (Figure 1D). Interlobular septa and small vessels were not included in the lesion. The immunohistochemistry of the protein-like material revealed that KL-6 was stained diffusely and SP-A was positive in a fine granular pattern (Figure 2A,B). However, SP-D was negative (Figure 2C). The material was also positive for diastase-PAS, mucicarmine and alcian blue staining throughout the entire volume of the nodular GGO (Figure 2D,E,F). A serum anti-GM-CSF antibody test was negative and the KL-6 level was within the normal range (235 U/mL). Although genetic analysis was not carried out, the patient had no family history of PAP and had not taken any medications that could affect alveolar macrophage function. Three months after the resection, chest CT detected no recurrence.
Discussion
About 5% of mixed nodular GGOs resected without preoperative tissue are supposed to be benign lesions (15). In the relevant literature, localized PAP is extremely rare (10-13) and is not generally assumed as a differential diagnosis of mixed nodular GGOs. However, PAP should be considered in cases of cancer-negative specimens for which diagnosis is difficult. Measurement of serum anti-GM-CSF antibody levels is useful even in diagnosis of localized autoimmune PAP, since its titer does not correlate with severity of the clinical manifestations (16). In fact, several cases of anti-GM-CSF antibody-positive localized PAP have been reported (11-13). On the other hand, serum anti-GM-CSF antibody is negative in only 0.4% of Japanese idiopathic PAP (17). To the best of our knowledge, this manuscript is the first case of anti-GM-CSF antibody-negative localized PAP with focal mucinosis.
Within the range of our diagnostic examinations, underlying diseases known to induce secondary PAP were not observed in our case. In addition, tissue disorders such as fibrosis, organization, bleeding and inflammatory cell infiltration in the alveolar region, which are generally recognized in secondary PAP, were not found in this case (18). However, from a pathological point of view, there is a possibility that localized tissue damage of bronchiole due to unknown etiology induced epithelial hyperplasia. Since bacteriological examinations, such as tissue culture, were not carried out, local infection cannot be excluded.
KL-6 is mucinous high-molecular-weight glycoprotein and its levels in the serum and bronchoalveolar lavage fluid of PAP patients have been shown to be extremely high, correlating with disease activity (19,20). Previous studies indicated that KL-6 immunoreactivities were observed in type II pneumocytes in PAP patients, suggesting that KL-6 positive staining in lung tissue supports diagnosis of PAP (19,20). The intra-alveolar material in PAP was also positive for KL-6, as well as SP-A and SP-D: KL-6 and SP-A were localized in the intra-alveolar fine granular substances and SP-D was localized in the SP-A-negative foci corresponding to eosinophilic large globules (6,21). However, the staining patterns in our patient (KL-6, diffuse; SP-A, fine granular; SP-D, negative) were not consistent with those of previous cases, suggesting an unknown pathophysiology differing from typical PAP (6,21). Positive findings on diastase-PAS, mucicarmine and alcian blue staining indicated that the intra-alveolar material was mainly derived from epithelial mucus. We speculated that epithelial mucus produced by mucus-secreting cells in the epithelial hyperplasia of the bronchioles accumulated in the surrounding alveolar spaces, and that KL-6 and SP-A on the alveolar epithelium were incorporated into the mucus. Since mucus is positive for PAS staining, its accumulation in the alveolar region alone may appear to be a pathological image as PAP. However, it was unclear whether the accumulation of mucus was the main cause or secondary change of localized PAP in our patient.
ACCP guidelines for Asia define the diagnostic pathway depending on the size of the pulmonary nodule. In an individual with a solid nodule >8 mm in diameter and with moderate clinical probability of malignancy, 18F-FDG-PET is recommended, while in an individual with a part-solid nodule measuring >8 mm in diameter, the additional option of PET is recommended after a 3-month follow-up CT (4). On the other hand, PAPs with heterogeneous accumulation of 18F-FDG were reported, including a localized case (22,23). Therefore, it is difficult to suspect nodular PAP by noninvasive examination before surgical biopsy. The same guidelines recommend further evaluation with nonsurgical biopsy and/or surgical resection in a patient with suitable surgical risk for nodules persisting beyond 3 months, and our patient preferred to undergo video-assisted thoracoscopic partial resection of the right lower lobe. However, CT-TNB seemed to be easily incorporated in the presented case with a superficial lesion compared with surgical resection. In addition, a recent meta-analysis found that CT-TNB had a 26% better diagnostic yield [pooled diagnostic yield: 92%, 95% confidence interval (CI): 88–95] than transbronchial lung biopsy using radial endobronchial ultrasound and virtual bronchoscopic navigation (66%, 95% CI: 55–76) at tissue biopsy of small pulmonary lesions <2 cm in diameter, although complications of pneumothorax and hemorrhage were common with CT-TNB (24).
Conclusions
Solitary pulmonary nodule assessment may create unexpected difficulties. In the case of cancer-negative specimens, specific staining for PAP, including the atypical type with focal mucinosis, should be considered in differential diagnosis. In future research, it may be valuable to observe patients with localized PAP to propose an optimal diagnostic model, and to understand whether such cases are examples of a distinctive disease phenotype or an early finding of potentially progressing diffused disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Katki HA, Kovalchik SA, Petito LC, et al. Implications of Nine Risk Prediction Models for Selecting Ever-Smokers for Computed Tomography Lung Cancer Screening. Ann Intern Med 2018;169:10-9. [Crossref] [PubMed]
- Markaki M, Tsamardinos I, Langhammer A, et al. A Validated Clinical Risk Prediction Model for Lung Cancer in Smokers of All Ages and Exposure Types: A HUNT Study. EBioMedicine 2018;31:36-46. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-120S.
- Bai C, Choi CM, Chu CM, et al. Evaluation of Pulmonary Nodules: Clinical Practice Consensus Guidelines for Asia. Chest 2016;150:877-93. [Crossref] [PubMed]
- Wang BM, Stern EJ, Schmidt RA, et al. Diagnosing pulmonary alveolar proteinosis. A review and an update. Chest 1997;111:460-6. [Crossref] [PubMed]
- Kobayashi M, Takeuchi T, Ohtsuki Y. Differences in the immunolocalization of surfactant protein (SP)-A, SP-D, and KL-6 in pulmonary alveolar proteinosis. Pathol Int 2008;58:203-7. [Crossref] [PubMed]
- Borie R, Danel C, Debray MP, et al. Pulmonary alveolar proteinosis. Eur Respir Rev 2011;20:98-107. [Crossref] [PubMed]
- Mehrian P, Homayounfar N, Karimi MA, et al. Features of idiopathic pulmonary alveolar proteinosis in high resolution computed tomography. Pol J Radiol 2014;79:65-9. [Crossref] [PubMed]
- Kumar A, Abdelmalak B, Inoue Y, et al. Pulmonary alveolar proteinosis in adults: pathophysiology and clinical approach. Lancet Respir Med 2018;6:554-65. [Crossref] [PubMed]
- Norikane S, Kato K, Kojima K, et al. A case of pulmonary alveolar proteinosis that showed localized ground glass attenuation. Jpn J Clin Radiol 2008;53:660-3.
- Taniguchi H, Abo H, Touge M, et al. A case of idiopathic pulmonary alveolar proteinosis with multiple localized ground-glass opacities. Arerugi 2008;57:1061-6. [PubMed]
- Matsushima S, Yokomura K, Matsui T, et al. A case of pulmonary alveolar proteinosis presenting with miniscule ground-glass opacity in the apex of the left lung. Nihon Kokyuki Gakkai Zasshi 2011;49:553-7. [PubMed]
- Kojima K, Kato K, Fukazawa T, et al. A case of autoimmune pulmonary alveolar proteinosis appearing as a localized ground-glass opacity. Jpn J Radiol 2014;32:657-60. [Crossref] [PubMed]
- Hu H, Wang Q, Tang H, et al. Multi-slice computed tomography characteristics of solitary pulmonary ground-glass nodules: Differences between malignant and benign. Thorac Cancer 2016;7:80-7. [Crossref] [PubMed]
- Cho J, Ko SJ, Kim SJ, et al. Surgical resection of nodular ground-glass opacities without percutaneous needle aspiration or biopsy. BMC Cancer 2014;14:838. [Crossref] [PubMed]
- Seymour JF, Doyle IR, Nakata K, et al. Relationship of anti-GM-CSF antibody concentration, surfactant protein A and B levels, and serum LDH to pulmonary parameters and response to GM-CSF therapy in patients with idiopathic alveolar proteinosis. Thorax 2003;58:252-7. [Crossref] [PubMed]
- Inoue Y, Trapnell BC, Tazawa R, et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med 2008;177:752-62. [Crossref] [PubMed]
- Nakata K, Inoue Y, Takada T, et al. Clinical features of pulmonary alveolar proteinosis. THE LUNG perspectives 2007;15:59-63.
- Takahashi T, Munakata M, Suzuki I, et al. Serum and bronchoalveolar fluid KL-6 levels in patients with pulmonary alveolar proteinosis. Am J Respir Crit Care Med 1998;158:1294-8. [Crossref] [PubMed]
- Nakajima M, Manabe T, Niki Y, et al. Serum KL-6 level as a monitoring marker in a patient with pulmonary alveolar proteinosis. Thorax 1998;53:809-11. [Crossref] [PubMed]
- Brasch F, Birzele J, Ochs M, et al. Surfactant proteins in pulmonary alveolar proteinosis in adults. Eur Respir J 2004;24:426-35. [Crossref] [PubMed]
- Hsu CW, Liu FY, Wang CW, et al. F-18 FDG PET/CT in pulmonary alveolar proteinosis. Clin Nucl Med 2009;34:103-4. [Crossref] [PubMed]
- Wang YL, Fang N, Zeng L, et al. Localized Airspace Consolidation of Pulmonary Alveolar Proteinosis Mimicking Malignant Lesions in 18F-FDG PET/CT Imaging: One Case Report. Clin Nucl Med 2015;40:908-9. [Crossref] [PubMed]
- Han Y, Kim HJ, Kong KA, et al. Diagnosis of small pulmonary lesions by transbronchial lung biopsy with radial endobronchial ultrasound and virtual bronchoscopic navigation versus CT-guided transthoracic needle biopsy: A systematic review and meta-analysis. PLoS One 2018;13. [Crossref] [PubMed]


