Lung volume reduction surgery beyond the NETT selection criteria
Introduction
The National Emphysema Treatment Trial (NETT) ultimately proved on a high evidence-based level, that lung volume reduction surgery (LVRS) is very effective in selected patients (1,2). The study showed that not only dyspnea, lung function, exercise tolerance and quality of life improves, but it also prolongs survival compared to medical treatment. The best responders were patients with heterogeneous emphysema in the upper lobes and low exercise capacity. Several single center trials investigated LVRS in heterogeneous emphysema primarily in the upper lobes and reported excellent improvements suggesting that this indication is definitively established (3-7).
Nevertheless, the beneficial effect of volume reduction is to restore an emphysematous, overinflated lung to its “normal volume”. Reversion of hyperinflation also should work in different types of emphysema morphology. This article discusses LVRS in patients beyond the classical inclusion criteria defined by the NETT. They were defined as a result of their study for possible responders as symptomatic patients with certain lung function values: forced expiratory volume in one second (FEV1) lower than 45% predicted, total lung capacity (TLC) higher than 100% predicted and residual volume (RV) higher than 150% predicted. Previous LVRS, diffuse emphysema and pulmonary hypertension were exclusion criteria (1,8).
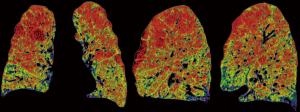
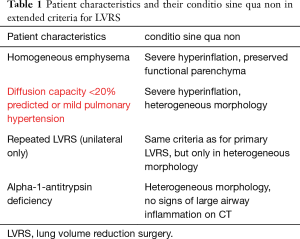
In the past years, we evaluated LVRS also for patients with different types of emphysema morphology on CT, patients with alpha1-antitrypsin deficiency (AATD), diffusion capacity values lower than 20% predicted or mild pulmonary hypertension, the latter ones uniquely in combination with heterogeneous emphysema and for patients who had repeated LVRS after successful previous surgical treatment (Table 1). Since LVRS is beside lung transplantation the most effective palliative treatment option in advanced emphysema patients, resulting in an increase of lung function and quality of life, as well as survival, widening the indication criteria beyond the upper-lobe predominant heterogeneous emphysema (Figure 1) seemed reasonable to us and we report on these wider indications.
Full table
Homogeneous emphysema
In the NETT, patients with all types of morphology and even in combination with very low FEV1 and/or low carbon monoxide diffusion factor (vanished lungs) were included and exposed to LVRS in order to find the limits of the procedure. Not surprisingly, the 69 patients with the combination of FEV1 less than 20% predicted and either homogeneous emphysema or low carbon monoxide diffusing capacity (DLCO) less than 20% predicted had a postoperative 30-day mortality rate of 16% (9). These criteria were previously used as exclusion criteria in most single center trials and became a rule for any further trials. This is for sound reasons since removing parenchyma that potentially contributes to gas exchange represents a reasonable concern in these patients with heavy destroyed lungs like the homogeneous emphysema type.
The main positive effect of LVRS is the improvement on respiratory mechanics after reshaping the overinflated lungs to its normal size (10,11). Therefore, carefully selected patients with homogeneous emphysema may also profit from LVRS (Figure 2). Our group was able to show sustained improvements after LVRS in a cohort of 138 patients with homogeneous emphysema (12). Their mean FEV1% predicted improved from preoperative 28% to 38% three months after LVRS. Hyperinflation (measured ratio of RV to TLC, RV/TLC) decreased significantly by 15% and diffusion capacity remained stable. Six-minute walking distance improved by 60 meters. The medical research council dyspnea score (MRCDS) was reduced from 3.5 to 1.8. The perioperative and the one-year survival did not differ between the 112 patients with heterogeneous emphysema operated during the same period. Subsequently, the latter had a slightly higher chance of transplantation-free survival, but improvements in lung function and exercise capacity lasted for a similar time (despite a smaller improvement at 3 and 6 months). Patients with homogeneous emphysema and destroyed lungs or a very low diffusion capacity or pulmonary hypertension should strictly not undergo LVRS. Morphology itself should not be an exclusion criterion. The key in selecting these patients for LVRS is severe hyperinflation (RV/TLC >60%) with “enough” preserved functional reserve (FEV1% and DLCO% both above 20).
Impaired diffusion capacity
Due to the high surgical mortality for patients with severely impaired diffusion capacity (DLCO <20%) in patients with homogeneous emphysema, these patients were excluded for LVRS in general. However, in heterogeneous emphysema, the resected tissue is functionless und will not further impair gas exchange, but the patient should profit from the effect of correcting hyperinflation. Recently published by our group (13) and already reported by Ciccone and Cooper in 2003 (5), patients with a diffusion capacity below 20% predicted were operated with zero mortality and a good profit. The 33 patients from Zurich showed preoperative pulmonary function values (FEV1, TLC, RV, RV/TLC) within the usual range for LVRS but had a severely impaired diffusion capacity (median 15%, interquartile range 13–18). Their lung function significantly improved three months after surgery (FEV1% predicted by 26%) and even the DLCO significantly increased by 60% and remained higher than pre-operative for the subsequent year (FEV1% predicted still 21% higher and DLCO 27% higher compared to preoperatively). The same beneficial effect of LVRS on lung function was true for the mentioned subgroup of 20 patients with DLCO <20% in the LVRS cohort from St. Louis. Possibly, severe hyperinflated parts compromise potentially functional pulmonary parenchyma on one hand and on the other hand the ventilation is improved. These parts are hypoventilated and regain their function after LVRS. Therefore, it seems reasonable and even logical to resect these functionless lung zones. Additionally, the more hyperinflated the patient the greater the effect can be expected. The most common complication after LVRS in patients with such a low DLCO is prolonged air leak lasting longer than 7 days. This might reflect the overall poor quality of these lungs and should be discussed with the patient preoperatively (13,14).
Repeated LVRS
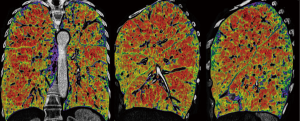
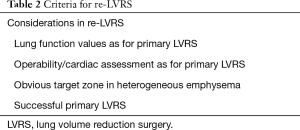
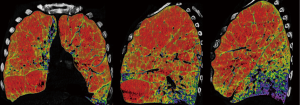
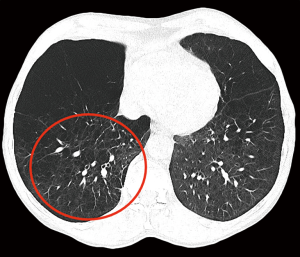
We have been performing repeated LVRS (re-LVRS) since 2002. In 2014, the results of 22 patients were published (15). While mean FEV1% predicted has improved from 27.8% to 45% after the first procedure, it improved from 28.6% to 34.8% three months after re-LVRS and was 31% twelve months after the second procedure. The MRC-dyspnea score decreased from 3.7 to 2.2 (3 months postoperatively) and remained improved after 1 year. 90-day mortality was zero. Since January 2014 another 19 patients underwent repeated LVRS at our institution and confirmed the initial results. Nevertheless, despite the zero perioperative mortality, postoperative morbidity is slightly higher than in primary LVRS. Patients had a significant longer drainage-time and hospital stay. Median chest tube time was eleven versus six days and in-hospital-time was 14 versus nine days. Other complications were rare and did not differ. Table 2 lists the essential considerations for re-LVRS. While pre-operative assessments do not differ, the patient should report a good benefit from the prior LVRS. However, patients would not be referred in case of a failed primary procedure. An obvious target zone for resection and a severe hyperinflation are of upmost importance. We perform re-LVRS procedures unilaterally because we want to keep a possible complicated postoperative course due to air leaks low and also the risk of resecting “too much” tissue must be minimal. Figure 3 shows the pre-operative CT-densitometry of a patient scheduled for re-LVRS. Despite the massively destroyed parts of the lungs, the patients still showed parts of lung parenchyma with a quite well preserved quality (Figure 4). Three and a half years ago, he underwent primary bilateral LVRS. FEV1 improved from 31% to 66% predicted three months postoperatively. The patient had a remarkable benefit, which declined especially over the last year. Now, preoperative lung function values were FEV1 24%, TLC 165%, RV 334% and RV/TLC 74%. The diffusion capacity was 30%. After unilaterally re-LVRS on the right side, postoperative lung function after 6 weeks already showed a FEV1 of 40% and a diffusion capacity of 40% and he had a major clinical improvement.
Full table
Pulmonary hypertension
Pulmonary hypertension was an absolute contraindication for LVRS in the NETT as well as in other studies. However, in combination with markedly heterogenous emphysema, where non-perfused lung tissues is resected, LVRS is not only improving dyspnea and lung function, but also pulmonary hemodynamics seem to be improved (16), which is further described in another article of the present issue (Opitz I et al.).
Alpha-1-antitrypsin-deficiency (AATD)
Ten patients with AATD underwent LVRS in the NETT (17).Two-year mortality was higher with LVRS compared with medical therapy (20% versus 0%). AATD-patients had lower and shorter durations of increases in FEV1 and exercise capacity compared with patients with LVRS without AAT deficiency. Our own results demonstrated clinical and physiologic improvements in lung function after LVRS in 21 patients with AATD as well. We observed an improvement that was maximal at three to six months and only slightly inferior to the one achieved in pure smoker’s emphysema. Durability of beneficial effects was between two and four years which is shorter than the usual time observed after LVRS (18). Nevertheless, a potential subgroup of AATD-patients with demonstrated long-term benefit was identified: patients with heterogeneous emphysema and no signs of chronic inflammation on CT scans (bronchiectasis, scarring) profit the most and possible transplantation can be successfully postponed with a good quality of life. Nevertheless, only little evidence exists in this field, but the concept of reducing hyperinflation and targeting heterogeneous emphysema areas seems to play the major role again.
Summary
LVRS works best in symptomatic patients with upper-lobe predominant emphysema and low exercise tolerance. However, it can be successfully performed in well selected patients with criteria beyond NETT. Patients with homogeneous emphysema, severely impaired diffusion capacity, mild pulmonary hypertension, a1-antitrypsin-deficiency and patients who already underwent LVRS may profit from surgical volume reduction as well. Selection, site and amount of resection require more experience. Several single-center series show good outcome in these subgroups of emphysema patients. Of course, single center series need interpretation with caution, but so far, better evidence as in the NETT is not and maybe never available for these highly selected subgroups. Decreasing the hyperinflation and the presence of heterogeneous emphysema morphology offering obvious target zones for resection in these even more risky candidates are the key issues.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. [Crossref] [PubMed]
- Criner GJ, Cordova F, Sternberg AL, et al. The National Emphysema Treatment Trial (NETT) Part II: Lessons learned about lung volume reduction surgery. Am J Respir Crit Care Med 2011;184:881-93. [Crossref] [PubMed]
- Brenner M, McKenna RJ Jr, Chen JC, et al. Relationship between amount of lung resected and outcome after lung volume reduction surgery. Ann Thorac Surg 2000;69:388-93. [Crossref] [PubMed]
- Flaherty KR, Kazerooni EA, Curtis JL, et al. Short-term and long-term outcomes after bilateral lung volume reduction surgery: prediction by quantitative CT. Chest 2001;119:1337-46. [Crossref] [PubMed]
- Ciccone AM, Meyers BF, Guthrie TJ, et al. Long-term outcome of bilateral lung volume reduction in 250 consecutive patients with emphysema. J Thorac Cardiovasc Surg 2003;125:513-25. [Crossref] [PubMed]
- Tutic M, Lardinois D, Imfeld S, et al. Lung-volume reduction surgery as an alternative or bridging procedure to lung transplantation. Ann Thorac Surg 2006;82:208-13; discussion 213. [Crossref] [PubMed]
- Ginsburg ME, Thomashow BM, Bulman WA, et al. The safety, efficacy, and durability of lung-volume reduction surgery: A 10-year experience. J Thorac Cardiovasc Surg 2016;151:717-724.e1. [Crossref] [PubMed]
- The Joint Commission on Certification in Lung Volume Reduction Surgery. Available online: https://www.jointcommission.org/certification/lung_volume_reduction_surgery.aspx, accessed 16.08.2018.
- National Emphysema Treatment Trial Research Group, Fishman A, Fessler H, et al. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001;345:1075-83. [Crossref] [PubMed]
- Cassart M, Hamacher J, Verbandt Y, et al. Effects of lung volume reduction surgery for emphysema on diaphragm dimensions and configuration. Am J Respir Crit Care Med 2001;163:1171-5. [Crossref] [PubMed]
- Sciurba FC, Rogers RM, Keenan RJ, et al. Improvement in pulmonary function and elastic recoil after lung-reduction surgery for diffuse emphysema. N Engl J Med 1996;334:1095-9. [Crossref] [PubMed]
- Weder W, Tutic M, Lardinois D, et al. Persistent benefit from lung volume reduction surgery in patients with homogeneous emphysema. Ann Thorac Surg 2009;87:229-36; discussion 236-7. [Crossref] [PubMed]
- Caviezel C, Schaffter N, Schneiter D, et al. Outcome After Lung Volume Reduction Surgery in Patients With Severely Impaired Diffusion Capacity. Ann Thorac Surg 2018;105:379-85. [Crossref] [PubMed]
- DeCamp MM, Blackstone EH, Naunheim KS, et al. Patient and surgical factors influencing air leak after lung volume reduction surgery: lessons learned from the National Emphysema Treatment Trial. Ann Thorac Surg 2006;82:197-206; discussion 206-7. [Crossref] [PubMed]
- Kostron A, Horn-Tutic M, Franzen D, et al. Repeated lung volume reduction surgery is successful in selected patients. Eur J Cardiothorac Surg 2015;48:710-5. [Crossref] [PubMed]
- Caviezel C, Aruldas C, Franzen D, et al. Lung volume reduction surgery in selected patients with emphysema and pulmonary hypertension. Eur J Cardiothorac Surg 2018;54:565-71. [Crossref] [PubMed]
- Stoller JK, Gildea TR, Ries AL, et al. Lung volume reduction surgery in patients with emphysema and alpha-1 antitrypsin deficiency. Ann Thorac Surg 2007;83:241-51. [Crossref] [PubMed]
- Bloch KE, Georgescu CL, Russi EW, et al. Gain and subsequent loss of lung function after lung volume reduction surgery in cases of severe emphysema with different morphologic patterns. J Thorac Cardiovasc Surg 2002;123:845-54. [Crossref] [PubMed]