Atrial septal defects and pulmonary arterial hypertension
Introduction
Atrial septal defects (ASDs) are the most common congenital heart disorder (CHD) after bicuspid aortic valve. They account for approximately 13% of patients with CHD (1). There is a female preponderance with a male-to-female ratio of 1:2. Most cases are usually sporadic, though genetic mutations have been implicated in some cases, particularly in the presence of other congenital defects and/or syndromes (2). The clinical consequence of these defects is related to the anatomic location, size and associated cardiac anomalies where present. For the majority of patients with the simplest ASD anatomy the clinical course is often benign, associated with a high probability of survival into adulthood (3). Nowadays, with advances in prenatal screening and fetal echocardiography, early life diagnosis allows for timely intervention.
Nevertheless, there remain a proportion of patients presenting in adult life for the first time. When diagnosed in adulthood, an ASD may have presented as an incidental finding. However, a proportion of patients present with symptoms such as breathlessness due to volume overload of the right ventricle (RV), right heart failure, arrhythmias, paradoxical embolism and occasionally pulmonary hypertension (PH).
The most common diagnostic tool is transthoracic echocardiography (TTE), which delineates the type of defect, size, and right ventricular function/volume overload status. 3D reconstruction on echocardiography is recommended when evaluating an ASD, as size may be underestimated on 2D transthoracic (TTE) or transoesophageal echocardiography (TOE) (Figure 1). Furthermore, additional non-invasive imaging such as cardiovascular magnetic resonance (CMR), or computed tomography (CT), are employed to exclude other commonly associated cardiac anomalies such as anomalous pulmonary venous drainage, for assessment of unexpected sinus venosus defect not seen on TTE or multiple ASDs (4).
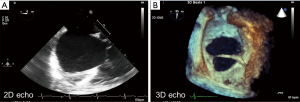
Sinus venosus ASD is occasionally first diagnosed at attendance for CMR for evaluation of PH (5,6). In cases that raise the suspicion of PH, either on echocardiography or clinically, right heart catheterization is necessitated to guide further management and intention of defect closure, if appropriate (7,8). If pulmonary arterial hypertension (PAH) is established, patients may no longer be suitable for defect closure and thus should follow an alternative line of management set out in the discussions to follow (9,10).
Types of ASDs
Anatomically, the ‘true’ atrial septum is the fossa ovalis, which covers the foramen ovale, whereas the remaining tissue separating the atria is composed of an in-folding of the atrial wall. A persistent defect at any level, in the fossa ovalis or along the remnant atrial walls, is known as an ASD, resulting in a direct communication between the two atrial chambers which allows for shunting of blood in either direction or even bi-directionally in some circumstances (11).
At the embryological stage of life, the fossa ovalis and thus atrial separation begins at approximately the fifth week of gestation. Two septa are involved in the closing of the orifice at an atrial level. Firstly, the septum primum arises from the superior portion of the common atrium and grows caudally eventually closing the ‘ostium primum’ orifice between the atria. Secondly, the septum secundum that arises from the right atrial side of the septum primum develops and covers the second orifice, the ostium secundum. Defects in the fossa ovalis are classified as secundum type ASDs. These defects account for over 70% of all ASDs. Larger secundum type ASDs can occur, and extend beyond the rims of the fossa ovalis, into the atrial walls, towards the inferior or superior vena cava (SVC). The majority of secundum type ASDs are isolated and rarely associated with anomalous pulmonary venous connections (12).
During intrauterine development the septum secundum does not completely cover the atria, leaving the foramen ovale patent, and only covered on the left side by the septum primum. At birth, as the lungs expand lowering pulmonary vascular resistance (PVR), there is a subsequent rise in the systemic vascular resistance, resulting in a reversal of the gradient at an atrial level and thus the septum primum is held against the septum secundum consequently closing the atrial shunt. If this process does not occur at birth, the result is a patent foramen ovale (PFO), with an estimated incidence of 25% to 30% in adults. Most patients are asymptomatic, though potentially PFOs may be implicated in a variety of clinical manifestations most importantly cryptogenic strokes (13).
Primum ASDs develop when the primum septum, as it grows caudally, fails to fuse with the endocardial cushions located between the atria and ventricles. The result is a defect at the base of the atrial septum, which is usually large. It is a commonly accepted that this type of lesion is part of an atrioventricular septal defect (AVSD), nearly always associated with anomalies of the atrioventricular valves, and contributes to either a partial (atrial component only) or complete (atrial and ventricular component) AVSD. This type of defect accounts for approximately 15% to 20% of ASDs and has more adverse long-term outcomes than secundum type ASDs (11).
Defects along the atrial wall but outside the rims of the fossa ovalis can also occur. Sinus venosus type ASD, inferior or superior, occurs when there is a failure of insertion of the atrial wall immediately below the orifice of the SVC or inferior (IVC) vena cava respectively. These defects account for 5% to 10% of atrial communication and are almost always associated with partial anomalous pulmonary venous connection. Inferior sinus venosus defects are rarer to superior and are more likely to be associated with right-to-left shunting and cyanosis than the former (14).
Sinus venosus defects are associated with anomalous pulmonary venous return to the SVC in the case of a superior sinus venosus defect and to the IVC in the case of an inferior sinus venosus defect.
The coronary sinus is the terminal vessel of the venous drainage of the heart, located posteriorly between the left atrium and ventricle, draining into the right atrium. A coronary sinus defect, otherwise known as an unroofed coronary sinus, occurs when part or the entire common wall between the coronary sinus and left atrium is absent. This defect is rare, and accounts for less than 1% of ASDs. It is often associated with a persistent left sided superior vena cava or complex congenital heart defects (15).
PH related to congenital heart disease (CHD)
PH is a clinical disorder, incited by an extensive heterogeneous number of pathophysiological triggers, resulting in a rise in pulmonary arterial pressures, PVR, subsequent right heart failure and premature death (16).
The haemodynamic definition of PH is a mean pulmonary arterial pressure (mPAP) of ≥25 mmHg at rest by means of right heart catheterization. More specifically, the definition of PAH, which describes a small proportion of patients displaying hemodynamic evidence of pre-capillary PH, whom, along with a mPAP ≥25 mmHg have the additional requisite of a pulmonary capillary wedge pressure (PCWP) of ≤15 mmHg, and a PVR of >3 Wood units (8).
The incidence of PAH related to CHD (PAH-CHD) varies geographically, but a consensus of registries globally estimates that up to 10% of adults with CHD develop PAH (17-19).
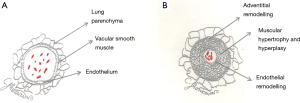
The pathophysiology in CHD, results from progressive vascular remodeling by a range of mechanisms depending on the underlying lesion. In PAH-CHD, often this is due to a defect with a left-to-right shunt large enough to allow sufficiently increased pulmonary blood flow to trigger a pathological mechanism whereby there is increased shear stress, endothelial dysfunction, smooth muscle hypertrophy, proliferation and progressive distortion of the pulmonary vasculature, thus contributing to the development of PAH (Figure 2). As the disease progresses, there is a consequent rise in the PVR, and if on right heart catheterization, this satisfies the hemodynamic definition of PAH, then the disease is established and irreversible, in most cases (8).
The extreme of this is when a significant left-to-right shunt continues to elevate the PVR to reach systemic levels; the shunt then reverses to a right-to-left or bi-directional status, thus leading to clinical cyanosis and the development of the Eisenmenger syndrome (ES). At present, the prevalence of ES ranges from 25% to 50% within the PAH-CHD cohort (18,20,21). Importantly, with advances in antenatal care and fetal screening, increasingly children are born into a prepared environment allowing for optimal timing of surgical intervention or are diagnosed at routine neonatal screening. Consequently, the pulmonary vasculature is protected from developing ES and the prevalence is reducing (16).
Classification of PAH-CHD
The classification of PH was first developed in 1998, categorizing groups that share similar pathological, hemodynamic characteristics and subsequent therapeutic approaches. The current classification system in use was finalized at the fifth World Health Organisation (WHO) meeting in 2013 and comprises of five groups. Group 1 is distinctly termed as PAH. PAH-CHD is categorized in Group 1, sharing hemodynamic features similar to idiopathic PAH, heritable PAH, PAH associated with other pathologies such as connective tissue disease, infections and drug-induced PAH (22). Furthermore, PAH-CHD is further described by two classification systems, one being a clinical classification system and the other is the anatomic-pathophysiological system (Table 1).

Full table
The clinical classification system divides the patients into four groups. Group 1 is patients with ES, characterized by a right-to-left shunt through a defect at any level, a significant elevation in PVR and cyanosis. ES is much less common in patients with an ASD than with a ventricular septal defect (VSD), nevertheless it is commonly seen due to the higher prevalence of ASDs (23). Group 2 are patients characterized by a left-to-right shunt through a moderate-to-large defect with a ‘mild-to-moderate’ elevation in PVR and cyanosis is not a feature. ASDs are the commonest in this group and although defect repair is often not suitable, evidence to guide the management in this group is limited (20). Group 3 are the PAH patients with a small/coincidental defect. These patients have a marked elevation in PVR, in the presence of an ASD <2 cm (or VSD <1 cm) which does not account for the development of the PAH. Often the clinical course and prognosis is similar to patients with idiopathic PAH and closure of the defect is contra-indicated. Group 4 is PAH after correction of the defect, either surgical or percutaneous. In these cases, the PAH either persists immediately after correction or develops months or years after the correction in the absence of a residual shunt. Often, the prognosis in this group is poorer after closure than if the lesion was left uncorrected (24).
The other classification system used in PAH-CHD is the anatomic-pathophysiologic classification. This system, categorizes lesions according to their anatomic location in relation to the tricuspid valve or complex anatomy. Pre-tricuspid defects include ASDs and anomalous pulmonary venous connections. Post-tricuspid lesions include VSDs and patent ductus arteriosus falls into this category. Lastly, complex defects include AVSDs, single ventricle physiology and some patients with large pre- and post-tricuspid defects.
In practice both systems are used by clinicians to differentiate patients with PAH-CHD. One study investigating the use of both classification schemes, showed that the anatomic-pathophysiologic classification was significantly better at differentiating survival compared to the clinical classification (25). This is further supported by other studies, which have shown that there is an adverse prognosis and outcome with pre-tricuspid lesions over post-tricuspid lesions (26). It has been implicated that in some pre-tricuspid lesions that are found in patients with PAH, may have a genetic disposition additional to their hemodynamic lesion further contributing to the pulmonary vascular remodeling and hence more aggressive disease, and may encourage more aggressive management of these patients (27). Nonetheless, CHD comprises of a very heterogeneous composition of patients, and some do not fit into a specific category, due to anatomical complexity.
Left-to-right ASDs and suitability for closure
Right heart catheterization is the gold standard investigation to confirm the diagnosis of PAH and furthermore, in patients with congenital shunts to support closure (8). As described above, some patients present with left-to-right shunts in whom the PVR measures ‘mild to moderately’ above the ‘safe to close’ threshold and thus present a management dilemma. A cautious approach is encouraged when evaluating patients with CHD defects and PH, moreover, identifying and closing a defect at the optimum time or not, is crucial.
In accordance with contemporary guidelines, if there is net left-to-right shunting (Qp:Qs >1.5), then a PVR <2.3 WU (PVRi <4 WU/m2) is deemed as a safe limit for shunt closure. If the PVR is >4.6 WU (PVRi >8 WU/m2), closure is not recommended. However, those that present with a PVR between 2.3–4.6 WU (PVRi 4–8 WU/m2) are less straightforward. Consideration needs to be made on a case-by-case basis. At times, one may need to consider that no intervention is better for the patient despite potentiating the risk of reversing the shunt in time to a right-to-left direction, and allowing the ES to ensue, albeit with significant morbidity and complications of a chronic cyanotic state, as this has a better long term prognosis than potentially erroneously closing the shunt and sacrificing the “relief valve” function of the ASD (24). Some strategies, suggest partial surgical closure or fenestrated or valved percutaneous closure allowing offloading/right atrial decompression in the case of an excessive rise in the PVR post repair (Figure 3) (28,29).
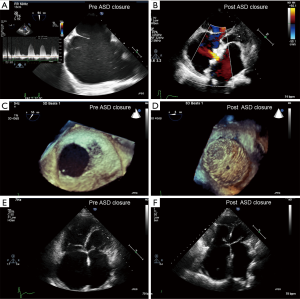
The “treat and repair” strategy has been attempted whereby patients are treated with pulmonary vasodilators and then undergo shunt closure at an interval after treatment (30). However, there is relative paucity of data on this strategy targeted for patients with borderline PVR or favorable reductions with vasoreactivity testing. Thus far, the data are limited to case reports or case series, therefore no clear consensus is available to guide clinicians (31).
Non-cardiac complications and management
Patients with ASDs developing PAH, have a clinical course and a series of complications that are variable and depend on the size, status of repair, and degrees of shunting in persistent defects. The most extreme form is the ES, which is characterized by multi-system involvement, precipitated by the chronic hypoxemia (32). Patients experience a vast spectrum of symptoms, particularly those with ES, which include dyspnea, fatigue, dizziness and headaches. Patient with ES, have a higher morbidity when compared to the general population and patients with non-cyanotic CHD albeit with longer survival rates compared to the other forms of PAH-CHD (19,33).
A series of specific complications and considerations are particular to patients with ES. They are comprised of both cardiac and non-cardiac complications. Although patients with ES can survive up to the sixth decade of life, they are burdened with a high level of morbidity and complexities related to their chronic hypoxemia.
Secondary erythrocytosis is a common feature in cyanosed patients and should be recognized as an adaptive and appropriate physiologic response that rarely requires intervention. Increased erythropoietin, secondary to the chronic hypoxemia, results in adjusted rises in the level of erythrocytes, thus manifesting as higher than ‘normal’ serum hemoglobin levels. Historically, patients were mismanaged with routine venesections to maintain a hematocrit level of <65% to reduce symptoms presumed to be secondary to hyperviscosity. Contemporary evidence-based knowledge, has confirmed this practice is not only unnecessary but also in fact harmful, by inducing iron deficiency anaemia (IDA), impairing oxygen transport capacity and reducing exercise tolerance (34). Rarely a venesection is indicated in the absence of dehydration or IDA, and must be done under experienced and expert supervision (35).
Other hemostatic abnormalities, seen in cyanotic patients include abnormalities of platelet production, platelet function and the coagulation pathway (36). Thrombocytopenia is common. Abnormal coagulation, as clotting factors is reduced and there is acquired depletion of von Willebrand factor, causes bleeding in these patients (37).
Patients with ES are at risk for both bleeding and thrombotic events. Hemoptysis is common and is often minor. Depending on the underlying etiology, interventional procedures such as embolization or coiling of bronchial arteries, may reduce recurrent events (38). Thrombosis, due to coagulopathy, atherosclerosis, and pro-thrombotic material or endothelial injury is also an important and observed complication. In situ thrombus, may develop along large, calcified and aneurysmal central pulmonary arteries in up to 30% of patients with ES, and is an indication for anticoagulation (39).
Unlike other types of PAH, patients with ES are not routinely anti-coagulated due to the bleeding tendencies. However, contemporary guidance encourages anticoagulation in patients with ES if there is an indication such as atrial arrhythmia or intrapulmonary thrombus is present (8).
IDA is an important and frequent complication seen in patients with ES and may be potentiated by bleeding, dietary intake, medication or hematological disorders. It occurs in between a third to a half of patients with ES (32). For cyanosed patients, the expected hemoglobin range is 18 to 24 g/dL, therefore observing clinicians should be wary of anaemia at hemoglobin levels <18 g/dL, in contrast to hemoglobin interpretation in the general population. The correction of IDA has been shown to improve morbidity factors such as exercise capacity and quality of life (40). Patients should be routinely monitored for IDA and it should be corrected with oral or intravenous supplementation.
Hyperuricemia is commonly seen in cyanosed adults and if symptomatic, due to gout, then uricosuric or uricostatic therapies are indicated. Asymptomatic hyperuricemia is not an indication for routine therapy. Patients with ES have a higher incidence of cholelithiasis due to the high red cell turnover. Management depends on the clinical presentation, but if necessary an open cholecystectomy is preferred over laparoscopic methods, as insufflation of carbon dioxide in the abdominal cavity may not be tolerated and must be performed in a centre experienced with PH.
Patients with any form PAH are decompensated by infections. Therefore, patients with fevers or other manifestations of infections should be investigated and treated promptly to avoid significant complications and potentially fatal outcomes. Important infections often associated with patients with ES, include endocarditis, cerebral abscess or pneumonia. All patients with ES should be educated on meticulous dental hygiene and are advised to have antibiotic prophylaxis for invasive dental procedures (41).
Oxygen therapy has not been shown to have an impact on measured clinical outcomes such as exercise capacity, hematological profiles, quality of life or survival (42). It is prescribed to patients for symptomatic relief, particularly those with end-stage disease or awaiting transplant surgery. With regards to air travel, oxygen supplementation may be administered but is not routinely required. Air travel, is deemed safe as long as airplanes are adequately pressurized (43,44).
Pregnancy is contraindicated for women with all forms of PAH, and is associated with a high morbidity and mortality, whereby pregnancies are complicated by cardiac, obstetric and fetal complications (45). Women who become pregnant are strongly advised on early termination of pregnancy, and if the patient is amenable should be performed in a centre with experience in PH. Fully informed women who decide to continue with their pregnancy, despite the risks, require careful and close follow up and monitoring throughout the pregnancy. Teratogenic medications such as ERAs and other cardiovascular supporting therapies should be discontinued for the duration of the pregnancy. PDE-5i and prostacyclin analogues have been used in pregnancy with adequate safety profiles for the fetus. For best outcomes, mothers should be managed by a multi-disciplinary approach in experienced centers, with a period of close observation for up to 2 weeks after the delivery (46). Contraceptive methods should be discussed with all female patients with PAH-CHD. Estrogen containing formulations are contraindicated due to the increased risk of thromboembolism. ERAs, particularly bosentan, may reduce the efficacy of oral contraceptives, therefore women are advised to use the double contraception approach with barrier methods to ensure efficacy. Intra-uterine devices and sterilization are also acceptable options, but as they may induce vagal events or require an anesthesia, should be performed in experienced PH centres.
Cardiac complications and management
Morbidity and mortality in patients with PAH related to an ASD are often related to cardiac complications. Advanced heart failure and arrhythmias are the most common mode of death in this cohort. Measures to preserve RV function by escalating pulmonary vasodilators in a timely fashion are of paramount importance to a patient’s survival outcome.
Heart failure
The RV maladaptation to the increased afterload generated by the exaggerated PVR, results in RV distension and premature dysfunction. RV failure is a common and major determinant of morbidity and mortality in PAH. As the RV size increases, a paradoxical ‘squashing’ of the left ventricle (LV) associated with a reduced preload due to the high PVR, results in a low cardiac output state, further exacerbating the morbidity and mortality for these patients. The management of RV failure is challenging and limited to symptomatic relief with diuresis when pulmonary vasodilators have not rescued the RV (47,48). Established therapies proven to be of benefit in LV failure do not convey the same benefit in RV failure. Patients, who do present in overt heart failure, should be managed in a critical care environment, with early identification of precipitating factors and reversing or treating the underlying trigger. Preferred inotropes are the phosphodiesterase type 3 inhibitors. Milrinone is favored over β1 agonists (dobutamine and dopamine), which may induce more systemic vasodilation and require the addition of a vasoconstrictor (48). In end-stage RV failure, RV assist devices are not an option as their function depends on favorable and low PVR, therefore if appropriate extracorporeal life support, when all other measure have failed, may be considered as a bridge to transplant (49).
Arrhythmias
Arrhythmias are a common and important complication in PAH, and can be associated with a clinical deterioration resulting in hospitalization and death (50). Supraventricular tachycardias are more common due to atrial distension or previous scar, and are an indication for anti-coagulation for patients with ES (32). Identified arrhythmias should be treated promptly in an appropriate setting if the patient shows signs of decompensation. Alternatively, rate and rhythm controlling medication such as amiodarone are favored over β-blockers, which may be poorly tolerated due to the negative isotropy. Catheter ablation should be considered in cases of recurrent atrial arrhythmias (51,52). Malignant ventricular arrhythmias are less often seen. However, if present, extrinsic coronary artery compression due to PA enlargement, electrolyte imbalances or an acquired channelopathy should be ruled out. Implantable defibrillators may be considered for secondary prevention, however the risk of bleeding, infection and the use of sedation during the implantation should be considered when weighing up the risks and benefits (52).
Coronary compression
Pulmonary artery dilatation is a common manifestation for patients with PAH-CHD. Pulmonary artery dilatation my result in dissection or rupture, and is often a terminal event. The left main coronary artery (LMCA) arises from the left coronary cusp of the aortic valve and runs an epicardial course branching out into the left anterior descending artery and the left circumflex artery which supply much of the LV, atrium and interventricular septum. As the pulmonary artery enlarges due to the underlying pathological process of PAH, it may dilate extensively such that extrinsic compression of the LMCA is caused. This can manifest as symptoms similar to an acute coronary syndrome and may even present as an ST elevation myocardial infarction if the extrinsic compression is severe. The treatment is often percutaneous intervention with a covered bare metal or drug eluting stents, even if there is no atherosclerotic disease. Other possible options, albeit rarely performed, include coronary artery bypass grafting and heart-lung transplantation (53).
Advanced therapies
The direct treatment of PAH requires the use of specific disease targeting pulmonary vasodilators, however, these are not curative and remain a “palliative” measure in this aggressive disease process. Targeted pulmonary vasodilators have only been in clinical use since the 1990s and in this short space of time; only three pathological routes have been translated into clinical practice, namely phosphodiesterase inhibitors/guanylate cyclase stimulators, endothelin receptor antagonists (ERA) and prostacyclin analogues/prostacyclin receptor agonists. Evidence based data supporting their use in patients with PAH related to an ASD be it ES or other form of PAH-CHD, is derived from data conducted on a majority patient population of idiopathic PAH and a handful of randomized controlled trials (RCT) specifically investigating pulmonary vasodilators on patients with ES (8,54,55).
Algorithms for the commencement and addition of second or third line pulmonary vasodilators, in patients with PAH related to their ASD vary depending on the underlying group of PAH-CHD. Patients with ASD deemed to have a coincidental small ASD or that have PAH post repair of their ASD are often treated more aggressively than patients with ES. The most recent joint European Society of Cardiology/European Respiratory Society guidelines on the management of PH now support the use of upfront combination therapy in newly diagnosed patients rather than the historical sequential mode of therapy long used (8). However, these guidelines broadly apply to patients that behave more like idiopathic PAH, who populate most of the clinical trials, and which include PAH with a small coincidental ASD (less than 2 cm) or post repair of an ASD defect. Patients with ES are often treated on a case-by-case practice, by a multi-disciplinary panel of experienced clinicians. Moreover, the use of pulmonary vasodilators in patients with ES has been shown to have a survival benefit when compared to patients that are treatment naïve, thus adequate treatment should be encouraged in this cohort and escalated promptly when patients show a clinical decline (27,56).
Phosphodiesterase type 5 inhibitors and guanylate cyclase stimulators
Phosphodiesterase type 5 inhibitors (PDE-5i) are a group of enzymes that inactivate the cyclic adenosine monophosphate (cAMP) and guanosine monophosphate (cGMP), which are upregulated in patients with PAH, thus resulting in vasodilatation in areas they are most dense (57). Tadalafil and sildenafil are the oral preparations of these inhibitors most often used in patients with PAH. There uses in patients with ES are supported by two RCTs (n=48), which demonstrated improvements in exercise capacity, functional class, invasive pulmonary artery pressures and echocardiographic parameters (58,59). PDE-5i is often used in combination therapy with an ERA; its use as single agent in patients with PAH is reducing.
Riociguat is a new therapeutic agent slowing down the degradation of cGMP degradation and thus potentiating vasodilatation has recently emerged. Riociguat is a guanylate cyclase stimulator (sGC) and its safety and efficacy in repaired CHD patients has been encouraging (60). However, there is no published data on its use in patients with ES.
ERA
Endothelin-1, mediated through receptors ETA and ETB, is a powerful vasoconstrictor, which is up-regulated in patients with PAH and induces pathological proliferation, fibrosis and inflammation. Three commercially available ERAs are in use, namely ambrisentan, a single receptor (ETA) antagonist, bosentan and macitentan that target both ETA and ETB receptors. Bosentan, the oldest ERA in use is supported positively by RCT data in patients with ES, and is currently endorsed as a first line treatment strategy in this cohort (61). Ambrisentan, which has a longer half-life, has shown favorable outcomes in patients with ES by means of prospective studies however the RCT did not include patients with PAH-CHD (62,63). Macitentan, is the latest ERA available, boasting dual ET receptor antagonism with longer affinity for the receptors than bosentan. The SERAPHIN trial showed reduced morbidity, mortality and improved exercise tolerance in forms of PAH, including post repair PAH-CHD (64). The MAESTRO trial of macitentan on walk distance is the largest RCT conducted on patients with ES (n=226) and has recently been completed, the results of which are pending (65). ERAs are an important therapeutic strategy in patients with PAH-CHD, including patients with ES, and are routinely offered as first line or in combination with a PDE-5i as endorsed consensus guidelines (8). As there is a low risk of hepatotoxicity or anaemia, patients require regular blood test monitoring.
Prostacyclin analogues and prostacyclin receptor agonists
Prostacyclin is produced mainly in endothelial cells and is a potent vasodilator by stimulating the production of cAMP and inhibiting the growth of smooth-muscle cells (66). One of the pathological routes involved in PAH is dysregulation of the prostacyclin pathway. Prostacyclin analogues are the most potent vasodilator and the first therapy to have had a significantly favorable mortality outcome in patients with idiopathic PAH. Its parenteral route of administration, intravenous, inhaled or subcutaneous, can influences patient’s willingness to initiate and then comply with therapy. The only orally available prostacyclin analogue, beraprost, is not widely used possibly due to the high frequency of unfavourable side effects. Overall, there is limited evidence on the use of prostanoids in patients with PAH-CHD. It is often reserved as a third line, palliative or bridge to transplant strategy in PAH-CHD, particularly patients with ES.
Selexipag, a selective IP receptor agonist, with a mode of action similar to prostacyclin, is one of the newest oral preparations for PAH. An RCT, included up to 10% of post-repair PAH-CHD, showed that in combination with an ERA and/or PDE-5i reduced morbidity and mortality (67). Its use is quite novel and as yet, no published literature is available on its use in patients in ES. As the general consensus, supported by RCT data, moves towards aggressive upfront dual or triple combination therapy, selexipag due to its enteral route is easier to endure than alternative parenteral prostacyclin analogues.
Prognosis and risk evaluation
A risk assessment table is available, featuring a series of relevant determinants of prognosis, which if present render a patient low, intermediate or high risk. These correlate to a <5%, 5–10% or >10% 1-year mortality, respectively (8). Although it is widely used to stratify all patients with PAH, the caveat is that it has only been validated in mostly idiopathic PAH, thus the cut-offs may not apply to other forms of PAH. Given the anatomical and physiological complexity of ES, Gatzoulis et al., purposely adapted the table for patients with ES citing determinants of ‘better’ and ‘worse’ prognosis in this cohort (68). Furthermore, in a detailed study of over 1,000 adults with ES, a multivariate risk stratification model was produced, confirming that age, pre-tricuspid shunt, lower resting oxygen saturations, absence of sinus rhythm and the presence of a pericardial effusion were significant predictors of death (27). Such risk stratification tables and evidence of significant predictors of adverse outcomes, support clinicians in timely escalation or up titration of pulmonary vasodilators.
Conclusions
ASDs are less commonly associated with significant PH. Suitability for ASD closure when PVR is between 2.3 and 4.6 WU (PVRi 4–8 WU/m2) is not straightforward and clinical decision-making is individualized. Considerations include, whether to intervene with a complete defect closure, fenestrated closure or the ‘treat and repair’ strategy may be debated. When PH is significant despite a small ASD or following timely repair of ASD it may be regarded as coincidental. PH with a small/repaired ASD has a worse prognosis than unrepaired ASD. ES is rarely related to an ASD, and when present associated with an adverse prognosis compared to post tricuspid lesions. Overall, PAH associated with an ASD is associated with a high morbidity and mortality; patients should be monitored closely in specialized tertiary centres by a multi-disciplinary team approach to achieve optimum outcomes.
Acknowledgements
Funding: SV Babu-Narayan is supported by the British Heart Foundation (FS/11/38/28864).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 2011;58:2241-7. [Crossref] [PubMed]
- Posch MG, Perrot A, Berger F, et al. Molecular genetics of congenital atrial septal defects. Clin Res Cardiol 2010;99:137-47. [Crossref] [PubMed]
- Moons P, Bovijn L, Budts W, et al. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation 2010;122:2264-72. [Crossref] [PubMed]
- Babu-Narayan SV, Giannakoulas G, Valente AM, et al. Imaging of congenital heart disease in adults. Eur Heart J 2016;37:1182-95. [Crossref] [PubMed]
- Swift AJ, Rajaram S, Campbell MJ, et al. Prognostic value of cardiovascular magnetic resonance imaging measurements corrected for age and sex in idiopathic pulmonary arterial hypertension. Circ Cardiovasc Imaging 2014;7:100-6. [Crossref] [PubMed]
- van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 2011;58:2511-9. [Crossref] [PubMed]
- Thomson LE, Crowley AL, Heitner JF, et al. Direct en face imaging of secundum atrial septal defects by velocity-encoded cardiovascular magnetic resonance in patients evaluated for possible transcatheter closure. Circ Cardiovasc Imaging 2008;1:31-40. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119. [Crossref] [PubMed]
- Moledina S, Pandya B, Bartsota M, et al. Prognostic significance of cardiac magnetic resonance imaging in children with pulmonary hypertension. Circ Cardiovasc Imaging 2013;6:407-14. [Crossref] [PubMed]
- Jensen AS, Broberg CS, Rydman R, et al. Impaired Right, Left, or Biventricular Function and Resting Oxygen Saturation Are Associated With Mortality in Eisenmenger Syndrome: A Clinical and Cardiovascular Magnetic Resonance Study. Circ Cardiovasc Imaging 2015;8. [Crossref] [PubMed]
- Gutgesell HP, Huhta JC. Cardiac septation in atrioventricular canal defect. J Am Coll Cardiol 1986;8:1421-4. [Crossref] [PubMed]
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation 2008;118:e714-833. [Crossref] [PubMed]
- Kerut EK, Norfleet WT, Plotnick GD, et al. Patent foramen ovale: a review of associated conditions and the impact of physiological size. J Am Coll Cardiol 2001;38:613-23. [Crossref] [PubMed]
- al Zaghal AM, Li J, Anderson RH, et al. Anatomical criteria for the diagnosis of sinus venosus defects. Heart 1997;78:298-304. [Crossref] [PubMed]
- Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation 2006;114:1645-53. [Crossref] [PubMed]
- Nashat H, Brida M, Price LS, et al. Pulmonary Arterial Hypertension Complicating Congenital Heart Disease: Advances in Therapy. Semin Respir Crit Care Med 2017;38:636-50. [Crossref] [PubMed]
- Duffels MG, Engelfriet PM, Berger RM, et al. Pulmonary arterial hypertension in congenital heart disease: an epidemiologic perspective from a Dutch registry. Int J Cardiol 2007;120:198-204. [Crossref] [PubMed]
- Engelfriet PM, Duffels MG, Moller T, et al. Pulmonary arterial hypertension in adults born with a heart septal defect: the Euro Heart Survey on adult congenital heart disease. Heart 2007;93:682-7. [Crossref] [PubMed]
- Dimopoulos K, Wort SJ, Gatzoulis MA. Pulmonary hypertension related to congenital heart disease: a call for action. Eur Heart J 2014;35:691-700. [Crossref] [PubMed]
- Galie N, Manes A, Palazzini M, et al. Management of pulmonary arterial hypertension associated with congenital systemic-to-pulmonary shunts and Eisenmenger's syndrome. Drugs 2008;68:1049-66. [Crossref] [PubMed]
- Alonso-Gonzalez R, Lopez-Guarch CJ, Subirana-Domenech MT, et al. Pulmonary hypertension and congenital heart disease: An insight from the REHAP National Registry. Int J Cardiol 2015;184:717-23. [Crossref] [PubMed]
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62:D34-41. [Crossref] [PubMed]
- Waddell TK, Bennett L, Kennedy R, et al. Heart-lung or lung transplantation for Eisenmenger syndrome. J Heart Lung Transplant 2002;21:731-7. [Crossref] [PubMed]
- van Loon RL, Roofthooft MT, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation 2011;124:1755-64. [Crossref] [PubMed]
- Ramjug S, Hussain N, Hurdman J, et al. Pulmonary arterial hypertension associated with congenital heart disease: Comparison of clinical and anatomic-pathophysiologic classification. J Heart Lung Transplant 2016;35:610-8. [Crossref] [PubMed]
- Moceri P, Kempny A, Liodakis E, et al. Physiological differences between various types of Eisenmenger syndrome and relation to outcome. Int J Cardiol 2015;179:455-60. [Crossref] [PubMed]
- Kempny A, Hjortshoj CS, Gu H, et al. Predictors of Death in Contemporary Adult Patients With Eisenmenger Syndrome: A Multicenter Study. Circulation 2017;135:1432-40. [Crossref] [PubMed]
- Talwar S, Choudhary SK, Saxena A, et al. Unidirectional valved patches for closure of septal defects in patients with severe pulmonary hypertension. Ann Pediatr Cardiol 2008;1:114-9. [Crossref] [PubMed]
- Althoff TF, Knebel F, Panda A, et al. Long-term follow-up of a fenestrated Amplatzer atrial septal occluder in pulmonary arterial hypertension. Chest 2008;133:283-5. [Crossref] [PubMed]
- Kijima Y, Akagi T, Takaya Y, et al. Treat and Repair Strategy in Patients With Atrial Septal Defect and Significant Pulmonary Arterial Hypertension. Circ J 2016;80:227-34. [Crossref] [PubMed]
- Dimopoulos K, Peset A, Gatzoulis MA. Evaluating operability in adults with congenital heart disease and the role of pretreatment with targeted pulmonary arterial hypertension therapy. Int J Cardiol 2008;129:163-71. [Crossref] [PubMed]
- Diller GP, Dimopoulos K, Broberg CS, et al. Presentation, survival prospects, and predictors of death in Eisenmenger syndrome: a combined retrospective and case-control study. Eur Heart J 2006;27:1737-42. [Crossref] [PubMed]
- Dimopoulos K, Okonko DO, Diller GP, et al. Abnormal ventilatory response to exercise in adults with congenital heart disease relates to cyanosis and predicts survival. Circulation 2006;113:2796-802. [Crossref] [PubMed]
- Broberg CS, Bax BE, Okonko DO, et al. Blood viscosity and its relationship to iron deficiency, symptoms, and exercise capacity in adults with cyanotic congenital heart disease. J Am Coll Cardiol 2006;48:356-65. [Crossref] [PubMed]
- Oechslin E, Mebus S, Schulze-Neick I, et al. The Adult Patient with Eisenmenger Syndrome: A Medical Update after Dana Point Part III: Specific Management and Surgical Aspects. Curr Cardiol Rev 2010;6:363-72. [Crossref] [PubMed]
- Martin-Garcia AC, Arachchillage DR, Kempny A, et al. Platelet count and mean platelet volume predict outcome in adults with Eisenmenger syndrome. Heart 2018;104:45-50. [Crossref] [PubMed]
- Perloff JK, Rosove MH, Child JS, et al. Adults with cyanotic congenital heart disease: hematologic management. Ann Intern Med 1988;109:406-13. [Crossref] [PubMed]
- Rasciti E, Sverzellati N, Silva M, et al. Bronchial artery embolization for the treatment of haemoptysis in pulmonary hypertension. Radiol Med 2017;122:257-64. [Crossref] [PubMed]
- Perloff JK, Hart EM, Greaves SM, et al. Proximal pulmonary arterial and intrapulmonary radiologic features of Eisenmenger syndrome and primary pulmonary hypertension. Am J Cardiol 2003;92:182-7. [Crossref] [PubMed]
- Tay EL, Peset A, Papaphylactou M, et al. Replacement therapy for iron deficiency improves exercise capacity and quality of life in patients with cyanotic congenital heart disease and/or the Eisenmenger syndrome. Int J Cardiol 2011;151:307-12. [Crossref] [PubMed]
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075-128. [Crossref] [PubMed]
- Sandoval J, Aguirre JS, Pulido T, et al. Nocturnal oxygen therapy in patients with the Eisenmenger syndrome. Am J Respir Crit Care Med 2001;164:1682-7. [Crossref] [PubMed]
- Harinck E, Hutter PA, Hoorntje TM, et al. Air travel and adults with cyanotic congenital heart disease. Circulation 1996;93:272-6. [Crossref] [PubMed]
- Broberg CS, Uebing A, Cuomo L, et al. Adult patients with Eisenmenger syndrome report flying safely on commercial airlines. Heart 2007;93:1599-603. [Crossref] [PubMed]
- Ladouceur M, Benoit L, Radojevic J, et al. Pregnancy outcomes in patients with pulmonary arterial hypertension associated with congenital heart disease. Heart 2017;103:287-92. [Crossref] [PubMed]
- Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J 2011;32:3147-97. [Crossref] [PubMed]
- Hoeper MM, Granton J. Intensive care unit management of patients with severe pulmonary hypertension and right heart failure. Am J Respir Crit Care Med 2011;184:1114-24. [Crossref] [PubMed]
- Price LC, Wort SJ, Finney SJ, et al. Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care 2010;14:R169. [Crossref] [PubMed]
- Punnoose L, Burkhoff D, Rich S, et al. Right ventricular assist device in end-stage pulmonary arterial hypertension: insights from a computational model of the cardiovascular system. Prog Cardiovasc Dis 2012;55:234-43.e2. [Crossref] [PubMed]
- Ruiz-Cano MJ, Gonzalez-Mansilla A, Escribano P, et al. Clinical implications of supraventricular arrhythmias in patients with severe pulmonary arterial hypertension. Int J Cardiol 2011;146:105-6. [Crossref] [PubMed]
- Showkathali R, Tayebjee MH, Grapsa J, et al. Right atrial flutter isthmus ablation is feasible and results in acute clinical improvement in patients with persistent atrial flutter and severe pulmonary arterial hypertension. Int J Cardiol 2011;149:279-80. [Crossref] [PubMed]
- Hernández-Madrid A, Paul T, Abrams D, et al. Arrhythmias in congenital heart disease: a position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) Working Group on Grown-up Congenital heart disease, endorsed by HRS, PACES, APHRS, and SOLAECE. Europace 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Lee MS, Oyama J, Bhatia R, et al. Left main coronary artery compression from pulmonary artery enlargement due to pulmonary hypertension: a contemporary review and argument for percutaneous revascularization. Catheter Cardiovasc Interv 2010;76:543-50. [Crossref] [PubMed]
- Galiè N, Beghetti M, Gatzoulis MA, et al. Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation 2006;114:48-54. [Crossref] [PubMed]
- Hjortshøj CS, Jensen AS, Sondergaard L. Advanced Therapy in Eisenmenger Syndrome: A Systematic Review. Cardiol Rev 2017;25:126-32. [Crossref] [PubMed]
- Dimopoulos K, Inuzuka R, Goletto S, et al. Improved survival among patients with Eisenmenger syndrome receiving advanced therapy for pulmonary arterial hypertension. Circulation 2010;121:20-5. [Crossref] [PubMed]
- Humbert M, Lau EM, Montani D, et al. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation 2014;130:2189-208. [Crossref] [PubMed]
- Mukhopadhyay S, Nathani S, Yusuf J, et al. Clinical efficacy of phosphodiesterase-5 inhibitor tadalafil in Eisenmenger syndrome--a randomized, placebo-controlled, double-blind crossover study. Congenit Heart Dis 2011;6:424-31. [Crossref] [PubMed]
- Singh TP, Rohit M, Grover A, et al. A randomized, placebo-controlled, double-blind, crossover study to evaluate the efficacy of oral sildenafil therapy in severe pulmonary artery hypertension. Am Heart J 2006;151:851.e1-5. [Crossref] [PubMed]
- Rosenkranz S, Ghofrani HA, Beghetti M, et al. Riociguat for pulmonary arterial hypertension associated with congenital heart disease. Heart 2015;101:1792-9. [Crossref] [PubMed]
- Gatzoulis MA, Beghetti M, Galie N, et al. Longer-term bosentan therapy improves functional capacity in Eisenmenger syndrome: results of the BREATHE-5 open-label extension study. Int J Cardiol 2008;127:27-32. [Crossref] [PubMed]
- Galiè N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation 2008;117:3010-9. [Crossref] [PubMed]
- Zuckerman WA, Leaderer D, Rowan CA, et al. Ambrisentan for pulmonary arterial hypertension due to congenital heart disease. Am J Cardiol 2011;107:1381-5. [Crossref] [PubMed]
- Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013;369:809-18. [Crossref] [PubMed]
- Gatzoulis MA, Landzberg M, Beghetti M, et al. Evaluation of Macitentan in Patients with Eisenmenger Syndrome: Results from the Randomized, Controlled MAESTRO Study. Circulation 2018. In press.
- Jones DA, Benjamin CW, Linseman DA. Activation of thromboxane and prostacyclin receptors elicits opposing effects on vascular smooth muscle cell growth and mitogen-activated protein kinase signaling cascades. Mol Pharmacol 1995;48:890-6. [PubMed]
- Sitbon O, Channick R, Chin KM, et al. Selexipag for the Treatment of Pulmonary Arterial Hypertension. N Engl J Med 2015;373:2522-33. [Crossref] [PubMed]
- Gatzoulis MA, Beghetti M, Landzberg MJ, et al. Pulmonary arterial hypertension associated with congenital heart disease: recent advances and future directions. Int J Cardiol 2014;177:340-7. [Crossref] [PubMed]


