No-touch saphenous vein as an important conduit of choice in coronary bypass surgery
Coronary heart disease is a major cause of death and disability in developed countries. Coronary artery bypass grafting (CABG) remains the most effective way of treatment for patients with advanced disease (1). Approximately one million people undergo CABG annually worldwide, depicting the epidemiologic impact of the procedure and its influence on public health. Continuous efforts are employed to optimize the procedure of CABG. Long-term results depend highly on the appropriate conduit choice for bypass. Several groups support the use of multiple arterial grafts for improving long-term outcomes. Others claim a lack of unequivocal evidence that this strategy is associated with better clinical outcome and higher patency rates. The most widespread combination of grafts for CABG remains the left internal thoracic artery to the left anterior descending coronary artery supplemented with multiple venous grafts for remaining territories (2). However, relative to arterial grafts, saphenous vein graft failure rates were reported high, at up to 25% in the first 18 months (3).
Both radial and right internal thoracic artery grafts have been investigated and generally show better patency than saphenous-vein grafts. However, they are not routinely used due to the complexity of CABG with multiple arterial grafts. The use of a left internal thoracic artery graft to the left anterior descending coronary artery is considered a major quality indicator in CABG and the associated clinical outcomes are better than those of patients with no left internal thoracic artery graft (4). The excellent long-term results of the left internal thoracic artery have motivated surgeons to use both internal thoracic arteries. However, randomized trial data of a potential survival benefit with the bilateral internal thoracic artery approach is lacking (5,6). Also, it is a more complex procedure, associated with a higher risk of sternal wound complications (5). On the other hand, radial artery grafts are being increasingly used. While long-term patency of the radial artery has been established (7), the clinical benefit of the radial artery reported in observational studies has not been confirmed in a randomized clinical trial (8).
Patient selection is crucial for achieving excellent long-term results with the radial artery. Harvesting of the radial artery a priori requires evaluation for adequate ulnar artery flow. If ulnar artery flow is inadequate the radial artery should not be used in order to prevent the loss of hand function. Target vessel stenosis and run-off are key factors that determine radial artery patency. General agreement is that the radial artery should only be used to bypass target vessels with highly significant stenosis (9). Target vessel stenosis of >70% on the circumflex territory and >90% on the right coronary territory are considered suitable for bypassing with the radial artery. Surgeons are often confronted with borderline flow limiting stenosis where the radial artery was shown not to be effective. Contemporary practice entails increasingly difficult cases, older, obese and diabetic patients and those with small vessels and diffuse disease. In such cases performing sequential grafts seems to be the solution. Among all of the grafts used in CABG the radial artery is particularly prone to spasm, possibly because of its large medial cross-sectional area. Spasm has been reported to occur in 4–10% of radial grafts immediately after surgery (10). When the radial artery is used for CABG calcium channel blockers are needed to prevent spasm. Radial artery grafts should be avoided in patients with chronic renal disease, since harvesting of a radial artery in such patients precludes use of the ipsilateral arm for vascular access in case of later onset hemodialysis.
In a recent issue of The New England Journal of Medicine, the RADIAL (Radial Artery Database International ALliance) group provide evidence supporting the superiority of the radial artery when compared to the saphenous vein as a conduit of second choice in CABG (11). The authors performed a patient-level combined analysis to overcome the power limitations of individual underpowered studies. After performing a systematic literature search, they include six randomized controlled trials that compare clinical outcome and angiographic patency of the two grafts in CABG. By pooling the data from the six trials 1,036 patients were analyzed. The primary outcome was a composite of major adverse cardiac events during follow-up and included death, myocardial infarction, and repeat revascularization. The secondary outcome was graft patency at the protocol-defined follow-up angiography. After a mean follow-up of 5 years, the use of radial artery grafts was associated with a lower composite outcome of death, myocardial infarction, or repeat revascularization (hazard ratio 0.67; 95% confidence interval, 0.49–0.90, P=0.01) and with lower risk of two individual components, myocardial infarction and repeat revascularization, but not death from any cause. The use of radial-artery grafts was also associated with higher rates of angiographic patency.
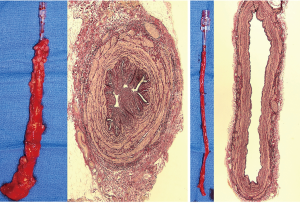
In spite of the excellent long-term angiographic results of the radial artery the need to improve the quality of saphenous vein grafts persists. The saphenous vein is still used in over 90% of CABG cases (2). Several attempts for improving the quality of saphenous vein grafts have been made. The ‘no-touch’ saphenous vein harvesting technique dramatically improves the long-term patency of saphenous vein grafts. Most of the studies that compared the saphenous vein to the radial artery patency employed the harvesting technique as originally described by Favaloro 50 years ago. Unlike the conventional technique, the ‘no-touch’ technique of saphenous vein harvesting leaves a pedicle of perivascular tissue intact, preserves the outer layers of the vessel wall, and obviates the need for graft distension (12). This technique improved long-term saphenous vein patency (13,14). The veins harvested using the ‘no-touch’ technique (Figure 1) have a patency rate of 83% at 16 years (16), while conventional saphenous vein grafts fail at a rate as high as 25% in the first 18 months (3). It has also been suggested that in elderly patients with multiple comorbidities the ‘no-touch’ saphenous graft could be used as a promising substitute for the left internal thoracic artery (17).
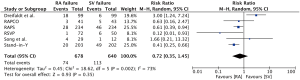
Some limitations of the graft patency analysis in the study by the RADIAL group need to be mentioned here (11). Due to absence of per-protocol angiography in one and within patient randomization in another trial, only four out of six trials were included in the main graft patency analysis. A total of 345 of 434 patients (79%) in the radial artery and 307 of 402 patients (76%) in the saphenous vein group underwent protocol defined angiography. A comparison of the baseline characteristics between patients with follow-up angiographic data and those without revealed significant baseline differences for age, sex, left ventricular ejection fraction, and target vessel revascularization. The mean follow-up time to protocol angiography was 50±30 months, with a range spanning from 1 to 143 months. Events of graft occlusion per 1,000 patient-years showed that the radial-artery grafts were associated with a significantly lower risk of occlusion (19 vs. 46; hazard ratio 0.44; 95% confidence interval, 0.28–0.70, P<0.001). Supplementary Appendix provides angiographic results by trial for five trials with protocol-driven angiography and overall, again revealing significantly lower risk of radial artery graft occlusion (10% vs. 19%; hazard ratio 0.46; 95% confidence interval, 0.31–0.69, P<0.001). The Örebro group compared the ‘no-touch’ saphenous vein and the radial artery patency in a controlled, randomized trial (18). This trial revealed that the ‘no-touch’ saphenous vein had a superior patency than the radial artery after 3-year follow-up. In spite of conducting a thorough literature search for publications comparing radial artery and saphenous vein grafts in CABG the study from the Örebro group, published in 2013 in The Annals of Thoracic Surgery (18), was not included in the analysis by the RADIAL group (11). Interestingly, if the angiographic patency data of the five trials with protocol-driven angiography were supplemented with the data from the Örebro group the difference in risk of graft occlusion between the radial artery and saphenous vein grafts dissipates (11% vs. 18%, risk ratio 0.72; 95% confidence interval, 0.35–1.45, P=0.35, I2=73%, Figure 2). The study by Song and colleagues, which was included in the RADIAL group analysis, employed the ‘no-touch’ harvesting technique per protocol (19). Out of the six trials included in the analysis, only this trial had numerically lower patency of the radial artery than the saphenous vein (11).
Intraoperative graft handling is of utmost importance for long-term graft quality, not only for saphenous vein but also for arterial grafts. The radial artery was introduced in the 70s, although it was soon abandoned because of early graft failure (20). After some modifications in practice (refined harvesting, routine calcium channel blocker administration, and careful choice of coronary targets) the radial artery was reintroduced in the 90s. Better understanding of radial artery biology was important for the improved results after the initial disappointing results (21). Conventional saphenous vein harvesting has not undergone significant refinement since its introduction. When the saphenous vein is harvested in the conventional manner it is stripped of its surrounding tissues, flushed with heparinized saline and manually distended with high pressures. The pressures that can easily be obtained with manual distension reach about 2.5 atmospheres, or 2,000 mmHg (22). These maneuvers are detrimental for the quality of saphenous vein grafts (15,23). Endoscopic vein harvesting may be considered as a major development for saphenous vein grafts in CABG. The advantage in terms of wound infection, wound healing, and scarring has resulted in the recent adoption of endoscopic vein harvesting, although issues have been raised regarding the patency of these grafts (24). The ‘no-touch’ technique of saphenous vein harvesting was introduced in 1996 and long-term results are promising (16). However, there remain concerns about increased leg-wound infection with ‘no-touch’ saphenous vein harvesting. Wound complications are directly related to the patients’ general condition and to the surgical technique. There are other important steps besides merely harvesting the vein with a fat pedicle such as ultrasonographic Doppler assessment which is used to mark the course of the vein. This facilitates the skin incision exactly above the vein, reducing soft tissue injury and creation of a flap. Besides, it allows selection of the best segment suitable for grafting. We recommend closing of the wound as soon as possible without leaving any dead space. To date, we have used ‘no-touch’ saphenous vein grafts in more than 3,000 patients, and, by implementing the technique as described previously (25), wound healing complications were considerably reduced. An ideal saphenous vein conduit would combine minimal risk of infection and excellent long-term performance. Therefore, to achieve a wide adoption of the technique a minimally invasive technique for harvesting the ‘no-touch’ saphenous vein grafts needs to be developed.
During the past decade there has been an almost 30% decline in CABG procedures in the United States, despite the supporting evidence for its use. Great advances in percutaneous coronary intervention was partially driven by the poor outcome when using saphenous vein grafts. However, ‘no-touch’ saphenous vein grafts are excellent conduits and, when used for CABG, ‘no-touch’ saphenous vein harvesting should be made a quality indicator. We would suggest, for those surgeons who are proponents of arterial conduits, that they firstly choose the target vessels that are suitable for arterial conduits, and then, if necessary, that they use ‘no-touch’ saphenous vein grafts for the remaining targets.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Head SJ, Milojevic M, Daemen J, et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet 2018;391:939-48. [Crossref] [PubMed]
- Schwann TA, Habib RH, Wallace A, et al. Operative Outcomes of Multiple-Arterial Versus Single-Arterial Coronary Bypass Grafting. Ann Thorac Surg 2018;105:1109-19. [Crossref] [PubMed]
- Fitzgibbon GM, Kafka HP, Leach AJ, et al. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol 1996;28:616-26. [Crossref] [PubMed]
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the Internal-Mammary-Artery Graft on 10-Year Survival and Other Cardiac Events. N Engl J Med 1986;314:1-6. [Crossref] [PubMed]
- Taggart DP, Altman DG, Gray AM, et al. Randomized Trial of Bilateral versus Single Internal-Thoracic-Artery Grafts. N Engl J Med 2016;375:2540-9. [Crossref] [PubMed]
- Yi G, Shine B, Rehman SM, et al. Effect of bilateral internal mammary artery grafts on long-term survival: A meta-analysis approach. Circulation 2014;130:539-45. [Crossref] [PubMed]
- Tatoulis J, Buxton BF, Fuller JA, et al. Long-Term Patency of 1108 Radial Arterial-Coronary Angiograms Over 10 Years. Ann Thorac Surg 2009;88:23-9; discussion 29-30. [Crossref] [PubMed]
- Gaudino M, Taggart D, Suma H, et al. The Choice of Conduits in Coronary Artery Bypass Surgery. J Am Coll Cardiol 2015;66:1729-37. [Crossref] [PubMed]
- Gaudino M, Alessandrini F, Pragliola C, et al. Effect of target artery location and severity of stenosis on mid-term patency of aorta-anastomosed vs. internal thoracic artery-anastomosed radial artery grafts. Eur J Cardiothorac Surg 2004;25:424-8. [Crossref] [PubMed]
- Tatoulis J, Royse AG, Buxton BF, et al. The radial artery in coronary surgery: a 5-year experience—clinical and angiographic results. Ann Thorac Surg 2002;73:143-7; discussion 147-8. [Crossref] [PubMed]
- Gaudino M, Benedetto U, Fremes S, et al. Radial-Artery or Saphenous-Vein Grafts in Coronary-Artery Bypass Surgery. N Engl J Med 2018;378:2069-77. [Crossref] [PubMed]
- Souza D. A new no-touch preparation technique: Technical notes. Scand J Thorac Cardiovasc Surg 1996;30:41-4. [Crossref] [PubMed]
- Souza DS, Dashwood MR, Tsui JC, et al. Improved patency in vein grafts harvested with surrounding tissue: results of a randomized study using three harvesting techniques. Ann Thorac Surg 2002;73:1189-95. [Crossref] [PubMed]
- Souza DS, Johansson B, Bojö L, et al. Harvesting the saphenous vein with surrounding tissue for CABG provides long-term graft patency comparable to the left internal thoracic artery: Results of a randomized longitudinal trial. J Thorac Cardiovasc Surg 2006;132:373-8. [Crossref] [PubMed]
- Dashwood MR, Tsui JC. 'No-touch' saphenous vein harvesting improves graft performance in patients undergoing coronary artery bypass surgery: a journey from bedside to bench. Vascul Pharmacol 2013;58:240-50. [Crossref] [PubMed]
- Samano N, Geijer H, Liden M, et al. The no-touch saphenous vein for coronary artery bypass grafting maintains a patency, after 16 years, comparable to the left internal thoracic artery: A randomized trial. J Thorac Cardiovasc Surg 2015;150:880-8. [Crossref] [PubMed]
- Samano N, Geijer H, Bodin L, et al. The no-touch saphenous vein graft in elderly coronary bypass patients with multiple comorbidities is a promising conduit to substitute the left internal thoracic artery. J Thorac Cardiovasc Surg 2017;154:457-66.e3. [Crossref] [PubMed]
- Dreifaldt M, Mannion JD, Bodin L, et al. The no-touch saphenous vein as the preferred second conduit for coronary artery bypass grafting. Ann Thorac Surg 2013;96:105-11. [Crossref] [PubMed]
- Song SW, Sul SY, Lee HJ, et al. Comparison of the Radial Artery and Saphenous Vein as Composite Grafts in Off-Pump Coronary Artery Bypass Grafting in Elderly Patients: A Randomized Controlled Trial. Korean Circ J 2012;42:107-12. [Crossref] [PubMed]
- Fisk RL, Brooks CH, Callaghan JC, et al. Experience with the Radial Artery Graft for Coronary Artery Bypass. Ann Thorac Surg 1976;21:513-8. [Crossref] [PubMed]
- Acar C, Jebara VA, Portoghese M, et al. Revival of the radial artery for coronary artery bypass grafting. Ann Thorac Surg 1992;54:652-9; discussion 659-60. [Crossref] [PubMed]
- Angelini G. Surgical Interventions for Veins [Internet]. [cited 2018 Jul 30]. Available online: http://www.ctsnet.org/article/surgical-interventions-veins?utm_source=iContact&utm_medium=email&utm_campaign=CTSNet&utm_content=Pulse+11%2F15%2F16
- Dashwood MR, Savage K, Tsui JCS, et al. Retaining perivascular tissue of human saphenous vein grafts protects against surgical and distension-induced damage and preserves endothelial nitric oxide synthase and nitric oxide synthase activity. J Thorac Cardiovasc Surg 2009;138:334-40. [Crossref] [PubMed]
- Kopjar T, Dashwood MR. Endoscopic versus “no-touch” saphenous vein harvesting for coronary artery bypass grafting. Angiology 2016;67:121-32. [Crossref] [PubMed]
- Souza DS, Arbeus M, Botelho Pinheiro B, et al. The no-touch technique of harvesting the saphenous vein for coronary artery bypass grafting surgery. Multimed Man Cardiothorac Surg 2009;2009:mmcts.2008.003624.


