Post-resection complications: abscesses, empyemas, bronchopleural fistulas
Introduction
Upper respiratory and pulmonary infections are common in the United States, and thoracic surgeons may be involved for both diagnostic and management purposes. Pneumonia is an acute pulmonary infection, and may be commonly caused by viruses and bacteria, and less commonly by fungi, mycoplasma, and parasites (Table 1). Centers for Disease Control (CDC) data from 2014 identify influenza and pneumonia as the primary cause of death for over 55,000 or 2.9% of all deaths during that period, making it the 8th leading cause of death in the United States (1). Mortality rates increase based on infection contraction location (hospital vs. outpatient) and associated comorbidities, and can approach 40% in critically ill patients (2). While these infections are highly morbid in themselves, the acute inflammation and immune response predisposes patients to other infections that may require thoracic surgical intervention.
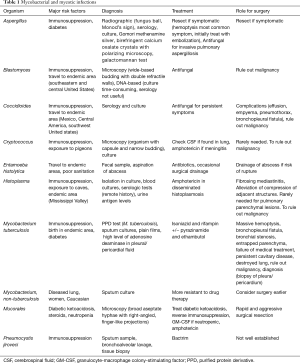
Full table
The majority of pulmonary infections are from inhalation sources though direct spread from adjacent tissue versus hematogenous spread is not uncommon (3). Pulmonary infections may remain confined to the lung and cleared by the immune system, or may become isolated and localized within lung parenchyma, causing a smoldering infection or abscess, or may spread beyond the lung parenchyma into the pleural space and cause an empyema. Abscesses are localized collections of purulent material contained within necrotic lung parenchyma, most frequently caused by aspiration of oral secretions (4). These historically required operative management to achieve source control; however, with modern antibiotic regimens, operation is usually now reserved for recalcitrant infections failing to improve with medical management (5). Effusions are collections of fluid within the pleural space, most often triggered by local inflammation from infection causing disruption in the lymphatic absorptive capacity of the lung, or post-operatively versus post-traumatic due to direct trauma to the visceral pleura (6). These can become infected, causing an empyema, or pleural space infection. These frequently require surgical management (7).
We herein review the historical and current guidelines for management of lung abscesses, empyemas, and bronchopleural fistulas (BPFs).
Abscess
Background
Lung abscesses are cavities within lung parenchyma containing debris and fluid as products of an infection. Historically, abscesses were a surgically-managed disease but now infrequently require operative control. Mortality rates prior to described surgical intervention were approximately 75% for untreated abscesses (8). Surgical management in the 1920’s and 1930’s was predicated on drainage and debridement of affected tissue—rib resection, aspiration of purulence to identify the abscess, then performing cutdown and debridement of vitalized tissue—and this decreased mortality to approximately 20–35% (8). However, with development of antibiotics in the 1940’s, and with interval improvements in therapy, surgical control is now only employed in about 10–15% of cases refractory to non-operative management (5,9-11).
Abscesses may be described as acute or chronic and have multiple risk factors (Table 2). Acute abscesses have symptoms of less than one month while chronic abscesses have symptom duration of over one month. Abscesses may also be described as primary or secondary. Primary abscesses, from direct inoculation of bacteria such as during aspiration events, represent 80% of abscesses (17) while secondary abscesses from underlying lung or systemic process, such as bronchial obstruction from cancer, septic emboli, and underlying lung problems like bronchiectasis, represent 20%. Organisms are often oral flora, and thus anaerobic and polymicrobial infections are common, each representing about 40% of all abscesses (12). Purely aerobic abscesses are less common and represent 10–15% of abscesses (10,12). Mortality remains elevated and highly variable, ranging from 5–75% in select patient series, especially with frequently nosocomial organisms such as Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas Aeruginosa though other factors predicting death include larger abscess size, underlying lung disease, neoplasms, altered mental status, immunocompromise, airway obstruction, and hemoglobin levels under 10 g/dL (13-16).
Diagnosis
The most typical presenting symptoms are cough, fever, and purulent sputum (18,19). Night sweats, weight loss, pleuritic chest pain, and hemoptysis are also commonly described symptoms (10,18,19). These symptoms may be difficult to distinguish from non-cavitary pulmonary infections. Cough with purulent sputum demonstrates communication of the abscess cavity with the bronchial tree, but is not specific to abscess formation (19). Depending on the organism and underlying health of the patient, the usual course is indolent and resembles pneumonia though some bacteria, most notably Staphylococcus aureus, are known to drive necrotizing pneumonia, which has an explosive course and may frequently require surgical source control if sepsis develops (20,21). Empyema is associated in one third of cases, typically from direct extension of the causative organism across the visceral pleura into the pleural space (22). BPF may be present in these cases of empyema (22). Other organisms, such as mycobacterial and mycotic infections, may also form abscesses though these are far less common.
The diagnosis is reinforced by imaging. The differential diagnosis of cavitary lung lesions includes solid pulmonary lesions or underlying malignancy (primary lung, lymphoma, Kaposi’s sarcoma), non-malignant cystic lesions (pulmonary sequestration, bronchial cysts), localized empyema, and granulomatous disease such as sarcoidosis and Wegener’s granulomatosis (15). Atypical causes include bronchiolitis obliterans organizing pneumonia and lung necrosis after pulmonary embolism (15). Computed tomography (CT) is the imaging modality of choice due to ability to demonstrate anatomy that cannot be seen in plain films such as small abscesses or solid lesions, differentiate abscess from empyema, and identify underlying causes for abscess (22,23). Chest radiographs may show a thick-walled cavity, occasionally with an air-fluid level, and are usually sufficient to initiate treatment (23). Specificity of chest radiographs to detect abscesses is, in some select patient series, similar to CT; however, sensitivity of CT is superior (24).
Sputum cultures and gram stain should be obtained prior to starting empiric therapy; however, these results may be easily misinterpreted due to oral flora contamination and cultures selectively favoring aerobic causes (25,26). Trans-tracheal aspirate, transthoracic biopsy, bronchoscopic aspiration, and bronchoalveolar lavage are other methods employed to obtain culture diagnosis (26,27) but are either not frequently used today nor not well validated. Pleural cultures and blood cultures are useful adjuncts in specific cases but unlikely to identify anaerobes after empiric treatment is started. Regular bronchoscopy has limited use for diagnosis; however, has use if there is concern for anatomic alterations or mass lesions. In addition, it can help identify causal factors for patients that do not respond appropriately to antibiotic therapy (28).
Medical treatment
Historically, surgical control was necessary; however, with advancement of antibiotic regimens, the majority of patients with bacterial and mycobacterial causes do not require surgical intervention. Multiple successful courses are described in the literature (25,29-37). Intravenous antibiotics with beta lactam/beta lactamase inhibitors or carbapenems, which have good lung penetration and broad activity against anaerobes, are preferred for most organisms (Table 3) (10,33-37). Therapy type and duration is frequently dictated by likely causal organisms, speciation and sensitivity data, and clinical response. Most patients will respond clinically within 7–10 days and can be transitioned to oral therapy until radiographic improvement is demonstrated (35).
Treatment for non-bacterial causes vary widely based on the organism. Fungi, amoeba, and parasite causes have varying susceptibility patterns. Mycobacterium tuberculosis (TB) is historically treated with quadruple therapy of rifampin, isoniazid, ethambutol, and pyrazinamide, though newer regimens are more common with emerging worldwide resistance (36).
Surgical treatment
Surgical control of infection is not as commonly employed anymore due to the success of antibiotic regimens. However, 10 to 15 percent of patients do not clear their infection using antibiotic therapy or recur after therapy completion, develop pulmonary hemorrhage, or have underlying suspected neoplasms, and for these patients’ surgical control is recommended (38,39). Other predictors for eventual need for operative source control include large abscesses at diagnosis (>6 cm), underlying lung disease such as severe chronic obstructive pulmonary disease (COPD), bronchiectasis, or obstructing lesions, particularly virulent pathogens causing necrotizing pneumonia, progressive hemoptysis, pulmonary sepsis, empyema with or without BPF (40,41). Studies have shown that concurrent empyemas increase intensive care unit admission rate, 30-day mortality, and overall mortality compared to patients with abscess alone (42). Resection is typically lobectomy or segmentectomy with the goal being to remove the abscess and surrounding necrotic tissue that remains a nidus for further infection. Pneumonectomy is rarely needed, with rare exceptions largely being necrotizing infections with entire lung involvement (8,41,43). Few reports exist since 1980 in these mortality remains in the 10–25% range (41,43-46).
There is significant disagreement on the best time to perform lung resection—many of these patients are debilitated from increased catabolic activity from the acute infection, and with delays caused by attempted medical management, patients lose additional reserve and ability to tolerate major thoracic surgery. Some studies have touted open drainage to achieve source control as superior to resection though with open drainage, often a second stage of resection is subsequently needed (44,47). Others have demonstrated no survival disadvantage from single-stage procedure (43,45).
Much of the recent work on techniques for lung resection focus on resections for bronchiectasis and mycobacterial infections, with these series containing some patients having developed abscesses (47-51). While the original standards of resection involved open thoracotomy, video-assisted thoracoscopic surgery (VATS) has proven safe for resection in prospective studies (41,50).
Percutaneous and endoscopic drainage is well described in the literature, and is an effective means of control, especially in patients who may not tolerate a lung resection, or have other significant medical comorbidities that increase risk of operative control (52). Decision to proceed with surgical resection vs drainage, however, lacks well-validated direct comparison, and requires assessment based on individual patient factors, hospital resources, and operator skill. Multiple limited studies have demonstrated utility, with an average success rate of 84%, complication rate of 16%, and mortality rate of 5% (5). Risks of percutaneous drainage include pneumothorax, hemothorax, hemoptysis. Current overall mortality rate appears to be under 4–13% when surgery is performed by experienced surgeons (5,53). The feared complication is creation of a BPF by the drain tract, by which bacteria can enter the pleural space, causing an empyema.
Empyema
Background
Empyemas are infected pleural effusions. Infection occurs when microorganisms, often bacteria, cross the pleural membrane in the context of an effusion, commonly from heart failure, cirrhosis, nephrotic syndrome, bacterial or viral pneumonia, post-operative, or post-traumatic causes (7). Risk factors share overlap with abscesses, and include diabetes mellitus, alcohol abuse, gastro-esophageal reflux, and immunosuppression (54). They are a major source of morbidity because, unlike abscesses which are predominantly managed with antibiotics as primary therapy, empyemas usually require surgical drainage in addition to antibiotics (55,56). Even with operative intervention, empyemas carry significant risk of mortality; in one study, even with drainage by tube thoracostomy, 10% of patients still died (55).
Empyemas progress along a well stereotyped pathway in three phases: exudative, fibrinopurulent, and organizing (57). Exudative empyemas represent the earliest stage of development, and are characterized by either serous or progressively more purulent fluid. Exudative empyemas may have an acidotic pH (<7.2), diminished glucose (<40 mg/dL), elevated lactate dehydrogenase (>1,000 IU/dL), elevated protein (>2.5 g/dL), elevated white blood cell count (>500/µL), and elevated specific gravity (>1.018) (57). Bacteria may be seen on culture, but this is typically associated with later stages of empyema development. All of these features define an effusion with decreased function of the pleural lining or bacterial presence. Fibrinopurulent empyemas represent the progression of exudative empyema, where the infection has progressed with subsequent inflammatory response and neutrophil recruitment. Thick purulent fluid may be encountered, and developing fibrin deposition from coagulation cascade activation occurs as the body attempts to wall-off and localize the infectious process. This causes the pleural space to septate, a feature which may be identified on imaging. Organizing empyema represents the final progression of this immune response, as the fibrin deposition forms a rind covering the visceral pleura. With remodeling of this rind, a thick capsule is formed and the lung becomes trapped (58,59).
Diagnosis
Unlike abscesses, empyemas are less frequently polymicrobial, and tend to be composed predominantly of aerobic bacterial causes (60-62). Obtaining pleural fluid samples is important to identify the characteristics and determine the nature of the fluid, so simultaneous gram stain and culture is feasible method for identifying the underlying organisms responsible. Aerobic and anaerobic cultures should be performed (60). Plain films may demonstrate a pleural effusion, but plural ultrasound is preferred as it allows for localization of collections prior to attempted aspiration (63). CT helps differentiate empyema from other lung disease and may be helpful from drainage but is not needed for initial evaluation given the utility of plain films and ultrasound.
Treatment
Empyemas, unlike abscesses, frequently require a surgical approach to control since the rapid progression and spread along the pleural space with fibrin deposition quickly causes a situation where antibiotic penetration is limited. Presence of frank pus or cloudy fluid on initial aspirate suggests bacterial infection and necessitates tube thoracostomy placement for source control (64). Antibiotics are used in combination with tube thoracostomy for initial management and source control and should be started as soon as infection is identified. Beta lactam/beta lactamase inhibitor therapy is recommended given the usual organism profile of an empyema, plus good penetration into the pleural space (63,64). In the setting of penicillin allergy, clindamycin may also be used (64).
Depending on the stage of the empyema, aspiration and/or chest tube placement alone may allow sufficient drainage for adequate antibiotic penetration and clearance of the infection. Open tube thoracostomy or image-directed placement of catheters are both well-accepted measures for treatment. For organized empyemas (fibrinopurulent or organizing), other factors such as loculations preventing adequate source control, persistent sepsis despite source control, and rind formation causing lung entrapment, are considerations for thoracoscopic versus open surgical management (65). Studies also show that administration of intrapleural tissue plasminogen activator (tPA) and DNase twice daily over 3 days via tube thoracostomy improves drainage and reduces need for surgical intervention, compared to chest tube and tPA or DNase monotherapy (65). In a study in which 210 patients with pleural infection were randomized one of 3 arms: (I) double placebo; (II) intrapleural tPA and DNase, and (III) tPA and placebo or DNA and placebo—frequency of surgical referral at 3 months was lower in the tPA-DNase group (2/48 patients, 4%) compared to the placebo group (8/51, 16%; P=0.003). Furthermore, tPA-DNase therapy was associated with a reduction in hospital stay compared to placebo (difference of 6.7 days, P=0.006). Chest tubes should be kept in place until clinical improvement of septic symptoms, and after confirmation of successful evacuation of the collection. For patients requiring operative intervention, VATS or open surgery may be performed for evacuation of all infected fluid plus excision of pleural rind and adhesions—VATS is usually better tolerated due to smaller incision size, and achieves similar results in terms of infection control and lung re-expansion (66). For patients with extensive involvement, failure to clear infection and/or chronic empyema, poor candidacy for major thoracic procedures such as decortication, creation of an Eloesser flap or Clagett window is an invasive but established option for control, and creates a large path for drainage, plus direct treatment of the involved pleural surfaces (67,68). A proposed algorithm for diagnosis and evaluation of empyema, along with lung abscesses, is proposed in Figure 1.
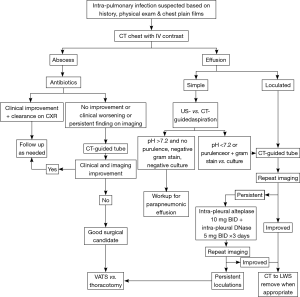
Broncho-pleural fistula
TB and atypical mycobacterial diseases
Due to an overall increase in the global incidence of TB, we have seen a resurgence of surgery in the management of TB. In addition, this is also due to an increase in human immunodeficiency virus incidence, improved survival of immuno-compromised patients, and the emergence of multi-drug-resistant TB (MDR-TB) since the mid-1980s.
A BPF is a serious and most feared complication after TB surgery. BPF in the post-operative setting indicates a breakdown of the bronchial stump. TB patients are at particular risk for BPF given their often poor nutritional status at the time of surgery and the usually already inflamed/infected bronchi. In reviewing large series (>20 patients) since 2000, BPF rate after lung resection for TB is in the range of 0–6.6% (Table 4) (69-82). There are no factors which have consistently been associated with BPF; however, some observations can be made. Bronchial stump reinforcement has been shown to decrease BPF in only one study (82). Despite the lack of clear evidence, most thoracic surgeons reinforce the bronchial stump with vascularized autografts, especially for pneumonectomy. The flaps can be fashioned from the pericardial fat pad, pleura, pedicled muscles such as intercostal, latissimus, or serratus, or omentum. The need for flaps highlights the importance of preoperative nutrition status. The type of closure (sutures versus stapler) also does not appear to be a risk factor. While peri-operative sputum positivity was found to be associated with increased BPF in one study (82), this also has not been found to be true in other studies since. In two studies, BPF was strictly seen in late post-operative period in the setting of disease relapse (70,79). This calls for strict control of disease peri- and post-operatively, especially in MDR-TB. In addition, in one study, endobronchial TB has been identified as a risk factor for BPF (72). For this reason, some surgeons perform frozen section to rule endobronchial TB at the time of resection.
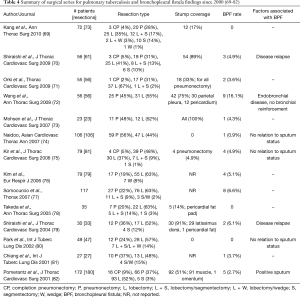
Full table
Conclusions
Here we present a review of historical and current management for surgically-treated pulmonary infections; lung abscesses, empyemas, and BPFs. As treatments continue to evolve and improve, the role of the surgeon for the management of pulmonary infection will also change. The skills needed to operate on these infections are the accepted technical standard for thoracic surgeons. Thus, in this evolving landscape of infection management, current knowledge of accepted and changing treatment practices is necessary for the thoracic surgeon to provide optimal care for patients with these significant, often life-threatening infections.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kochanek KD, Murphy SL, Xu JQ, et al. Deaths: Final data from 2014. National Vital Statistics Reports; vol 65 no 4. Accessed 2/08/2018. Available online: https://www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_04.pdf
- Leroy O, Santre C, Beuscart C, et al. A five-year study of severe community-acquired pneumonia with emphasis on prognosis in patients admitted to an intensive care unit. Intensive Care Med 1995;21:24-31. [Crossref] [PubMed]
- Torres A, Serra-Batlles J, Ferrer A, et al. Severe community-acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis 1991;144:312-8. [Crossref] [PubMed]
- Lorber B, Swenson RM. Bacteriology of aspiration pneumonia. A prospective study of community- and hospital-acquired cases. Ann Intern Med 1974;81:329-31. [Crossref] [PubMed]
- Wali SO. An update on the drainage of pyogenic lung abscesses. Ann Thorac Med 2012;7:3-7. [Crossref] [PubMed]
- Light RW, Girard WM, Jenkinson SG, et al. Parapneumonic effusions. Am J Med 1980;69:507-12. [Crossref] [PubMed]
- Farjah F, Symons RG, Krishnadasan B, et al. Management of pleural space infections: a population based analysis. J Thorac Cardiovasc Surg 2007;133:346-51. [Crossref] [PubMed]
- Schweigert M, Dubecz A, Stadlhuber RJ, et al. Modern history of surgical management of lung abscess: from Harold Neuhof to current concepts. Ann Thorac Surg 2011;92:2293-7. [Crossref] [PubMed]
- Schweppe HI, Knowles JH, Kane L. Lung abscess: An analysis of the Massachusetts General Hospital cases from 1943 through 1956. N Engl J Med 1961;265:1039-43. [Crossref] [PubMed]
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27-72. [Crossref] [PubMed]
- Takayanagi N, Kagiyama N, Ishiguro T, et al. Etiology and outcome of community-acquired lung abscess. Respiration 2010;80:98-105. [Crossref] [PubMed]
- Lorber B. Lung Abscess. In: Mandell G, Bennett J, Dolin R. editors. Principles and practice of infectious diseases. 6th edition. Churchill Livingstone, 2005:853-6.
- Magalhães L, Valadares D, Oliveira JR, et al. Lung abscesses: review of 60 cases. Rev Port Pneumol 2009;15:165-78. [PubMed]
- Gonҫalves AM, Menezes Falcão L, Ravara L. Pulmonary abscess: a revision. Rev Port Pneumol 2008;14:141-9. [PubMed]
- Ando K, Okhuni Y, Matsunuma R, et al. Prognostic lung abscess factors. Kansenshogaku Zasshi 2010;84:425-30. [Crossref] [PubMed]
- Mansharamani N, Balachandran D, Delaney D, et al. Lung abscess in adults: clinical comparison of immunocompromised to non-immunocompromised patients. Respir Med 2002;96:178-85. [Crossref] [PubMed]
- Bartlett JG. Anaerobic bacterial infections of the lung and plaural space. Clin Infect Dis 1993;16 Suppl 4:S248-55. [Crossref] [PubMed]
- Tsai YF, Ku YH. Necrotizing pneumonia: a rare complication of pneumonia requiring special consideration. Curr Opin Pulm Med 2012;18:246-52. [Crossref] [PubMed]
- Tristan A, Ferru T, Durang G, et al. Virulence determinants in community and hospital methicillin-resistant Staphylococcus aureus. J Hosp Infect 2007;65 Suppl 2:105-9. [Crossref] [PubMed]
- Williford ME, Godwin JD. Computed tomography of lung abscesses and empyema. Radiol Clin North Am 1983;21:575-83. [PubMed]
- Stark DD, Federle MP, Goodman PC, et al. Differentiating lung abscess and empyema: radiography and computed tomography. AJR Am J Roentgenol 1983;141:163-7. [Crossref] [PubMed]
- Schueller G, Matzek W, Kahns P, et al. Pulmonary infections in the late period after allogeneic bone marrow transplantation: chest radiography versus computed tomography. Eur J Radiol 2005;53:489-94. [Crossref] [PubMed]
- Bartlett JG. How important are anaerobic bacteria in aspiration pneumonia: when should they be treated and what is optimal therapy. Infect Dis Clin North Am 2013;27:149-55. [Crossref] [PubMed]
- Bartlett JG. Diagnostic accuracy of transtracheal aspiration bacteriologic studies. Am Rev Respir Dis 1977;115:777-82. [PubMed]
- Wimberley NW, Bass JB, Boyd BW, et al. Use of a bronchoscopic protected catheter brush for the diagnosis of pulmonary infections. Chest 1982;81:556-62. [Crossref] [PubMed]
- Sosenko A, Glassroth J. Fiberoptic bronchoscopy in the evaluation of lung abscesses. Chest 1985;87:489-94. [Crossref] [PubMed]
- Hecht DW. Anaerobes: antibiotic resistance, clinical significance, and the role of susceptibility testing. Anaerobe 2006;12:115-21. [Crossref] [PubMed]
- Weiss W. Oral antibiotic therapy of acute primary lung abscess: comparison of penicillin G and tetracycline. Curr Ther Res Clin Exp 1970;12:154-60. [PubMed]
- Levison ME, Mangura CT, Lorber B, et al. Clindamycin compared with penicillin for the treatment of anaerobic lung abscess. Ann Intern Med 1983;98:466-71. [Crossref] [PubMed]
- Gudiol F, Manresa F, Pallares R, et al. Clindamycin vs penicillin for anaerobic lung infections. High rate of penicillin failures associated with penicillin-resistant Bacteroides melaninogenicus. Arch Intern Med 1990;150:2525-9. [Crossref] [PubMed]
- Goldstein EJ, Citron DM, Warren Y, et al. In vitro activity of gemifloxacin (SB265805) against anaerobes. Antimicrob Agents Chemother 1999;43:2231-5. [Crossref] [PubMed]
- Allewelt M, Schuler P, Bolcskei PL, et al. Ampicillin + sulbactam vs clindamycin +/- cephalosporin for the treatment of aspiration pneumonia and primary lung abscess. Clin Microbiol Infect 2004;10:163-70. [Crossref] [PubMed]
- Appelbaum PC. Spangler Sk, Jacobs MR. Beta-lactamase production and susceptibilities to amoxicillin, amoxicillin-clavulanate, ticarcillin, ticarcillin-clavulanate, cefoxitin, imipenem, and metronidazole from 320 non-Bacteroides fragilis Bacteroides isolates and 129 fusobacteria from 28 U.S. Centers. Antimicrob Agents Chemother 1990;34:1546-50. [Crossref] [PubMed]
- Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 2012;54:621-9. [Crossref] [PubMed]
- Snydman DR, Jacobus NV, McDermott LA, et al. Lessons learned from the anaerobe survey: historical perspective and review of the most recent data (2005-2007). Clin Infect Dis 2010;50 Suppl1:S26-33. [Crossref] [PubMed]
- Tiberi S, Buchannan R, Caminero JA, et al. The challenge of the new tuberculosis drugs. Presse Med 2017;46:241-51. [Crossref]
- Bartlett JG. Lung abscesses and necrotizing pneumonia. In: Gorbach SL, Bartlett JG, Blackow NR. editors. Infectious Diseases. Philadelphia: W.B. Saunders, 1992.
- Schweigert M, Solymosi N, Dubecz A, et al. Predictors of Outcome in Modern Surgery for Lung Abscess. Thorac Cardiovasc Surg 2017;65:535-41. [Crossref] [PubMed]
- Mitchell JD, Yu JA, Bishop A, et al. Thoracoscopic lobectomy and segmentectomy for infectious lung disease. Ann Thorac Surg 2012;93:1033-9; discussion 1039-40. [Crossref] [PubMed]
- Huang HC, Chen HC, Fan HY, et al. Lung abscess predicts the surgical outcome in patients with pleural empyema. J Cardiothorac Surg 2010;5:88. [Crossref] [PubMed]
- Schweigert M, Dubecz A, Beron M, et al. Surgical therapy for necrotizing pneumonia and lung gangrene. Thorac Cardiovasc Surg 2013;61:636-41. [PubMed]
- Postma MH, Le Roux BT. The place of external drainage in the management of lung abscesses. S Afr J Surg 1986;24:156-8. [PubMed]
- Hagan JL, Hardy JD. Lung abscesses revisited. A survey of 184 cases. Ann Surg 1983;197:755-62. [Crossref] [PubMed]
- Delarue NC, Pearson FG, Nelems JM, et al. Lung abscess: surgical implications. Can J Surg 1980;23:297-302. [PubMed]
- Refaely Y, Weissberg D. Gangrene of the lung: treatment in two stages. Ann Thorac Surg 1997;64:970-3; discussion 973-4. [Crossref]
- Balkanli K, Genç O, Dakak M, et al. Surgical management of bronchiectasis: analysis and short-term results in 238 patients. Eur J Cardiothorac Surg 2003;24:699-702. [Crossref] [PubMed]
- Fujimoto T, Hillejan L, Stamatis G. Current strategy for surgical management of bronchiectasis. Ann Thorac Surg 2001;72:1711-5. [Crossref] [PubMed]
- Zhang P, Zhang F, Jiang S, et al. Video-assisted thoracic surgery for bronchiectasis. Ann Thorac Surg 2011;91:239-43. [Crossref] [PubMed]
- Mitchell JD, Bishop A, Cafaro A, et al. Anatomic lung resection for nontuberculous mycobacterial disease. Ann Thorac Surg 2008;85:1887-92. [Crossref] [PubMed]
- Herth F, Ernst A, Becker HD. Endoscopic drainage of lung abscesses: Technique and outcome. Chest 2005;127:1378-81. [PubMed]
- Hirshberg B, Sklair-Levi M, Nir-Paz R, et al. Factors predicting mortality of patients with lung abscess. Chest 1999;115:746-50. [Crossref] [PubMed]
- Pohlson EC, McNamara JJ, Char C, et al. Lung abscess: a changing pattern of the disease. Am J Surg 1985;150:97-101. [Crossref] [PubMed]
- Mengoli L. Giant lung abscess treated by tube thoracostomy. J Thorac Cardiovasc Surg 1985;90:186-94. [PubMed]
- Ferguson AD, Prescott RJ, Selkon JB, et al. The clinical course and management of thoracic empyema. QJM 1996;89:285-9. [Crossref] [PubMed]
- Davies CW, Kearney SE, Gleeson FV, et al. Predictors of outcome and long-term survival in patients with pleural infection. Am J Respir Crit Care Med 1999;160:1682-7. [Crossref] [PubMed]
- Huang HC, Chang HY, Chen CW, et al. Predicting factors for outcome of tube thoracostomy in complicated parapneumonic effusion for empyema. Chest 1999;115:751-6. [Crossref] [PubMed]
- Idell S, Girard W, Koenig KB, et al. Abnormalities of pathways of fibrin turnover in the human pleural space. Am Rev Respir Dis 1991;144:187-94. [Crossref] [PubMed]
- Brook I, Frazier EH. Aerobic and anaerobic microbiology of empyema. A retrospective review in two military hospitals. Chest 1993;103:1502-7. [Crossref] [PubMed]
- Chapman SJ, Davies RJ. The management of pleural space infections. Respirology 2004;9:4-11. [Crossref] [PubMed]
- Civen R, Jousimies-Somer H, Marina M, et al. A retrospective review of cases of anaerobic empyema and update of bacteriology. Clin Infect Dis 1995;20 Suppl 2:S224-9. [Crossref] [PubMed]
- Eibenberger KL, Dock WI, Ammann ME, et al. Quantification of pleural effusions: sonography vs radiology. Radiology 1994;191:681-4. [Crossref] [PubMed]
- Potts DE, Levin DC, Sahn SA. Pleural fluid pH in parapneumonic effusions. Chest 1976;70:328-31. [Crossref] [PubMed]
- Taryle DA, Good JT, Morgan EJ, et al. Antibiotic concentrations in human parapneuemonic effusions. J Antimicrob Chemother 1990;26:1-10.
- Teixeira LR, Villarino MA. Antibiotic treatment of patients with pneumonia and pleural effusion. Curr Opin Pulm Med 1998;4:230-4. [Crossref] [PubMed]
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNAse in pleural effusion. N Engl J Med 2011;365:518-26. [Crossref] [PubMed]
- Cardillo G, Carleo F, Carbone L, et al. Chronic postpneumonic pleural empyema: comparative merits of thoracoscopic versus open decortication. Eur J Cardiothorac Surg 2009;36:914-8. [Crossref] [PubMed]
- Thourani VH, Lancaster RT, Mansour KA, et al. Twenty-six years of experience with the modified eloesser flap. Ann Thorac Surg 2003;76:401-5. [Crossref] [PubMed]
- Bayes AJ, Wilson JA, Chiu RC, et al. Clagett open-window thoracostomy in patients with empyema who had and had not undergone pneumonectomy. Can J Surg 1987;30:329-31. [PubMed]
- Kang MW, Kim HK, Choi YS, et al. Surgical treatment for multidrug-resistant and extensive drug-resistant tuberculosis. Ann Thorac Surg 2010;89:1597-602. [Crossref] [PubMed]
- Shiraishi Y, Katsuragi N, Kita H, et al. Aggressive surgical treatment of multidrug-resistant tuberculosis. J Thorac Cardiovasc Surg 2009;138:1180-4. [Crossref] [PubMed]
- Orki A, Kosar A, Demirhan R, et al. The value of surgical resection in patients with multidrug resistant tuberculosis. Thorac Cardiovasc Surg 2009;57:222-5. [Crossref] [PubMed]
- Wang H, Lin H, Jiang G. Pulmonary resection in the treatment of multidrug-resistant tuberculosis: a retrospective study of 56 cases. Ann Thorac Surg 2008;86:1640-5. [Crossref] [PubMed]
- Mohsen T, Zeid A, Haj-Yahia S. Lobectomy or pneumonectomy for multidrug-resistant pulmonary tuberculosis can be performed with acceptable morbidity and mortality: a seven-year review of a single institution's experience. J Thorac Cardiovasc Surg 2007;134:194-8. [Crossref] [PubMed]
- Naidoo R. Active pulmonary tuberculosis: experience with resection in 106 cases. Asian Cardiovasc Thorac Ann 2007;15:134-8. [Crossref] [PubMed]
- Kir A, Inci I, Torun T, et al. Adjuvant resectional surgery improves cure rates in multidrug-resistant tuberculosis. J Thorac Cardiovasc Surg 2006;131:693-6. [Crossref] [PubMed]
- Kim HJ, Kang CH, Kim YT, et al. Prognostic factors for surgical resection in patients with multidrug-resistant tuberculosis. Eur Respir J 2006;28:576-80. [Crossref] [PubMed]
- Somocurcio JG, Sotomayor A, Shin S, et al. Surgery for patients with drug-resistant tuberculosis: report of 121 cases receiving community-based treatment in Lima, Peru. Thorax 2007;62:416-21. [Crossref] [PubMed]
- Takeda S, Maeda H, Hayakawa M, et al. Current surgical intervention for pulmonary tuberculosis. Ann Thorac Surg 2005;79:959-63. [Crossref] [PubMed]
- Shiraishi Y, Nakajima Y, Katsuragi N, et al. Resectional surgery combined with chemotherapy remains the treatment of choice for multidrug-resistant tuberculosis. J Thorac Cardiovasc Surg 2004;128:523-8. [Crossref] [PubMed]
- Park SK, Lee CM, Heu JP, et al. A retrospective study for the outcome of pulmonary resection in 49 patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2002;6:143-9. [PubMed]
- Chiang CY, Yu MC, Bai KJ, et al. Pulmonary resection in the treatment of patients with pulmonary multidrug-resistant tuberculosis in Taiwan. Int J Tuberc Lung Dis 2001;5:272-7. [PubMed]
- Pomerantz BJ, Cleveland JC Jr, Olson HK, et al. Pulmonary resection for multi-drug resistant tuberculosis. J Thorac Cardiovasc Surg 2001;121:448-53. [Crossref] [PubMed]




