Frozen elephant trunk with modified en bloc arch reconstruction and left subclavian transposition for chronic type A dissection
Introduction
Because of the high morbidity and mortality associated with acute type A dissection, only a small proportion of the patients with a delayed diagnosis and treatment survived into the chronic phase. However, chronic type A aortic dissection (CTAAD) is not uncommon in China owing to socio-economic reasons (1,2). In recent years, various approaches such as open surgical, hybrid (2-4) and endovascular repair (5,6) have been developed to treat CTAAD involving the aortic arch. Several techniques such as en bloc (island), branched graft (7,8) and branched stent graft (9) have been proposed for reconstruction of arch vessels during TAR. In our institution and other centers (1,4), total arch replacement (TAR) using a frozen elephant trunk (FET) is the procedure of choice to improve the long-term outcomes of patients with CTAAD. In the present study, we seek to review our experience with TAR and FET using a modified en bloc technique and left subclavian (LSCA)-left carotid artery (LCCA) transposition in patients with CTAAD during a 6-year period.
Methods
Patients
Between September 2010 and September 2016, 35 consecutive patients with CTAAD underwent surgical repair using en bloc technique with LSCA-LCCA transposition during the TAR and FET procedure under hypothermic circulatory arrest with selective antegrade cerebral perfusion (SACP). Mean age was 48.5±10.4 years (range, 28–70 years) and 29 were male (82.9%). Chronic aortic dissection was defined as a duration of >14 days from onset of symptoms to surgery (10). In this cohort, the median duration from symptom onset to surgery was 1.2 months (range, 0.5–84.0 months). Diagnosis was confirmed preoperatively by computed tomographic angiography (CTA) and echocardiography in all the patients.
Surgical technique
Our surgical technique of FET was previously described in detail (1,2,11). Briefly, right axillary artery cannulation was used for cardiopulmonary bypass (CPB) and SACP, heart was arrested with perfusion of cold blood cardioplegic solution, and unilateral SACP under hypothermic circulatory arrest at 25 °C was utilized with a flow rate of approximately 5–10 mL·kg–1·min–1. The procedure involved deployment of a FET (10 cm long and 24–30 mm in diameter), Cronus® (MicroPort Medical, Shanghai, China) in the true lumen between the origins of the LCCA and LSCA. Aortic valve or root procedures and concomitant operations were performed during the cooling phase.
The anterior wall of the aortic arch was incised longitudinally up to the origin of the LCCA, and the incision was about 5 mm distal to the origin of the innominate artery (IA) and LCCA. No dissection of the arch vessels was confirmed intraoperatively. After deployment of FET, the stent-free sewing edge (3 cm long Dacron graft) of the FET was straightened and trimmed to fit within native aortic wall containing the origins of the IA and LCCA. Next, the trimmed sewing edge was sutured to the native aortic wall near the origins of the IA and LCCA, as described in detail previously (12). Thus, the residual aortic arch wall and sewing edge formed a circular opening. Then, an end-to-end anastomosis was made between the rounded opening of the proximal aortic arch and distal ascending aortic graft. Subsequently, the flow rate was resumed to normal.
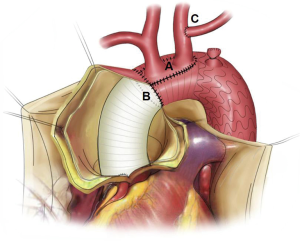
During the rewarming phase, the LSCA was clamped and transected 5–10 mm distal to its origin. The proximal stump of the LSCA was sewed with a running suture, and the distal end of the LSCA was anastomosed to the LCCA in an end-to-side fashion, as described previously (12,13). For patients with an ILVA, ILVA-LCCA transposition was performed in a similar way. These steps could be undertaken during the cooling phase if there was sufficient time. Figure 1 shows a schematic diagram of the modified procedure.
Patient follow-up
Operative survivors were followed up by clinic visits, telephone interviews, letters or emails. Complications such as neurologic and other morbidities were recorded. CTA was performed before discharge, at 3 and 6 months, 1 year and annually to evaluate thrombosis and obliteration of the false lumen, expansion of the true lumen, arch vessels patency, endoleak and other aortic complications.
Statistical analysis
Statistical analysis was performed using SPSS for Windows 19.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism for Windows 6.0 (GraphPad, La Jolla, CA, USA). Continuous variables were expressed as mean ± standard deviation (range); for variables not normally distributed, the median value was determined. Categorical variables are presented as number (percentage). Survival was estimated using the Kaplan-Meier method. Competing risks of death and reoperation were analyzed with the Fine and Gray proportional hazards model. All statistical tests were 2-sided and a P value of <0.05 was considered statistically significant.
Results
Preoperative data
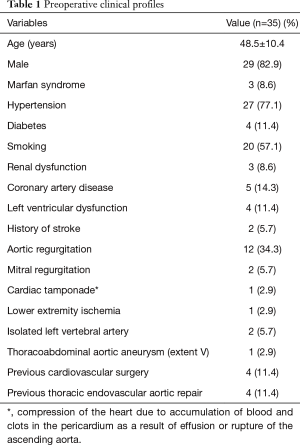
Comorbidities included hypertension in 27 patients (77.1%) and Marfan syndrome in 3 (8.6%). Preoperative renal dysfunction (serum creatinine level >1.5 mg/dL) was present in 3 patients (8.6%), left ventricular dysfunction (left ventricular ejection fraction <50%) in 4 (11.4%), and lower extremity ischemia in 1 (2.9%). Four patients (11.4%) had previous cardiac surgery, including Bentall procedure for type A dissection, aortic valve replacement, aortic and mitral valve replacement, and coronary artery bypass grafting (CABG) in 1 each. Four patients (11.4%) had previous thoracic endovascular aortic repair for type B dissection, 3 of them were retrograde type A dissection and 1 was a new type A dissection. An isolated left vertebral artery (ILVA) was noted in 2 patients (5.7%). Table 1 shows the preoperative characteristics of patients with CTAAD.
Full table
There was no dissection or aneurysm of the IA, LCCA, or distal LSCA among these patients. The primary tear was located in the ascending aorta in 21 patients (60.0%), transverse arch in 9 (25.7%), and proximal descending aorta in 5 (14.3%). Dissection extended to the distal aortic arch in 2 patients (5.7%), descending thoracic aorta in 4 (11.4%), abdominal aorta in 8 (22.9%) and iliac artery in 21 (60.0%), respectively. One patient had a Crawford extent V thoracoabdominal aortic aneurysm.
Surgical data
All patients underwent the procedure successfully. The average times of CPB, aortic cross-clamp, and SACP were 176±47 minutes (range, 111–268 minutes), 89±30 minutes (range, 49–189 minutes), and 29±6 minutes (range, 15–38 minutes), respectively; the mean operative time was 6.6±1.0 hours (range, 5.0–9.0 hours). The median amount of intraoperative blood transfusion was 4 units (range, 0–12 units) and fresh frozen plasma transfusion was 400 mL (range, 0–1,600 mL), respectively. Concomitant procedures are listed in Table 2.
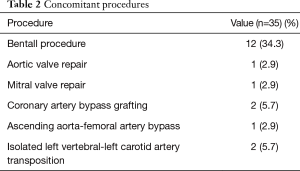
Full table
Operative morbidity and mortality
There were 2 in-hospital deaths (5.7%). One patient who underwent surgery 6 years after the initial diagnosis suffered ischemia in viscera organs and died of multiple-organ failure at postoperative 10 days; the other patient with a prior CABG underwent concomitant redo CABG and died of heart failure 9 days postoperatively. No neurological deficit or spinal cord ischemia occurred.
Re-exploration for bleeding was required in two patients (5.7%). Continuous renal replacement therapy was required temporarily in two patients with preoperative renal dysfunction (5.7%). Poor wound healing occurred in one patient (2.9%). All patients recovered uneventfully and were discharged from the hospital in a stable condition.
Follow-up
By March 2018, follow-up was complete in 100% (33/33) for a mean duration of 4.1±1.8 years (range, 0.5–6.7 years). One patient sustained a transient stroke and recovered after medication administration. One patient with Crawford extent V thoracoabdominal aortic aneurysm (6.5 cm in diameter) underwent planned thoracoabdominal aortic replacement 3 months after the TAR + FET procedure, and his postoperative course was uneventful. No cases of visceral malperfusion was observed during the follow-up period.
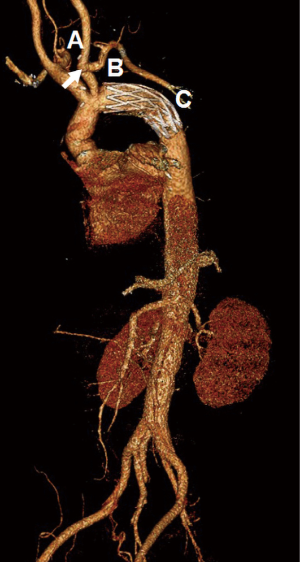
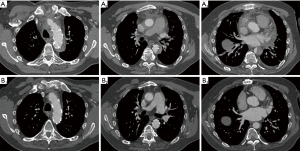
Follow-up CTA was available in 87.9% of patients (29/33). Persistent anastomotic leak of the aortic arch was observed in one patient (3.4%), but without false lumen expansion or pseudoaneurysm formation to the latest follow-up extending to 2 years. No stenosis or aneurysm of the anastomosis between the LSCA and LCCA was detected (Figure 2). Complete thrombosis, partial thrombosis and the false lumen patency around the FET were seen in 89.7% (26/29), 6.9% (2/29) and 3.4% (1/29), respectively. Complete thrombosis at the diaphragmatic level occurred in 62.1% (18/29) of patients (Figure 3).
Survival
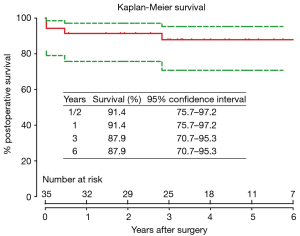
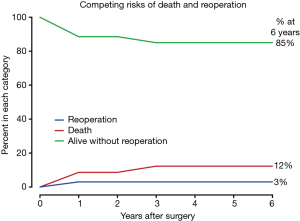
Two patients died during follow-up. One patient died of unknown reason at 6 months after ascending aorta replacement + TAR + FET + CABG; the other died of over-anticoagulation at 34 months after Bentall + TAR + FET + mitral valve repair. Overall survival was 91.4% (95% CI, 75.7–97.2%), 87.9% (95% CI, 70.7–95.3%) and 87.9% (95% CI, 70.7–95.3%) at 1, 3 and 6 years, respectively (Figure 4). In competing risks analysis, the incidence was 3% for reoperation at 6 years, 12% for late death, and 85% of patients were alive without reoperation (Figure 5).
Discussion
Acute type A aortic dissection is a lethal emergency associated with high mortality. Pathologic studies have shown that the aortic wall of the chronic dissection is relatively stable owing to increased thickness and stiffness of intimal flaps during the remodeling process (14,15). Hence, the mortality associated with type A dissection is significantly lower in the chronic phase (10). Currently, surgical management similar to that for acute type A dissection is accepted first-line treatment for CTAAD, with better outcomes than that for patients who undergo surgery in the acute phase (5,16). Various surgical techniques have been introduced to treat aortic arch pathologies of CTAAD (1,2,4,11,16), yet the optimal method is controversial. In recent years, the FET technique has offered a new alternative therapy for chronic and acute dissections. Several meta-analyses have revealed that the TAR and FET technique is associated with a lower prevalence of mortality, and reduces false lumen patency, and the need for reintervention compared with conventional arch procedures (17,18). In multicenter studies, respectable results have been achieved for CTAAD using the TAR and FET approach (1,3,4,19).
However, TAR remains a challenging surgical procedure and bleeding from the anastomoses remains a dreadful complication. Over the years, various techniques, such as the en bloc (island), branched graft (7,8), branched stent graft (9,20), and supra-aortic debranching (21,22), have been introduced to simplify the anastomoses and hemostasis during TAR. However, for the reimplantation of the arch vessels, the en bloc and branched graft are the most commonly used methods (7,8).
The classical en bloc technique needs only one anastomosis for the arch vessels and has the advantage of long-term patency by preserving the native arch vessels. However, this technique requires all the anastomoses to be completed before reperfusion can be resumed, which can prolong the cerebral and low body ischemia times. Meanwhile, the distal anastomosis in zone III (distal to the LSCA) and the hemostasis of the posterior part of the “island” are technically difficult because of the deep surgical field, which may increase the risk of anastomotic leaks. Moreover, there is a potential risk of aneurysmal dilation of the “island” containing a large piece of residual aortic wall.
In recent years, the branched graft technique has been applied widely during TAR in most aortic arch pathologies, and it has several advantages with respect to en bloc technique (8). Over the years, we have performed TAR using a 4-branched graft with the Cronus FET (the Sun procedure) for patients with complex chronic or acute type A dissection involving the arch or proximal descending aorta (23). The indications for this surgical procedure have been described in our previous studies, and favorable outcomes have been obtained in chronic patients (1,2,11). However, as many as 5 anastomoses need to be sutured during TAR in this procedure, which may prolong the hemostasis and operative times.
In addition, Shimamura et al. (20) and Chen et al. (9) have developed open double- or triple-branched stent graft for TAR in patients with type A dissection. Satisfactory short-term results have been achieved with these methods, but the postoperative migration and kinking of the branched graft may lead to endoleak or stroke (24). The supra-aortic debranching technique is also a promising alternative with the advantage of avoiding circulatory arrest (21,25). However, literature show that it is associated with a high rate of endoleak, stroke, and mortality (22,26). Other methods, such as trifurcated graft (27) and Y-graft (28), have been introduced, but their long-term outcomes for type A dissection remain known.
To address these issues, we have modified the en bloc technique for TAR and FET in type A dissection repair. In our modification, the “island” (residual aortic arch aortic wall containing only the IA and the LCCA origins) was trimmed into a very small piece, and all the native arch vessels were preserved via LSCA-LCCA transposition during TAR. The selection criteria of this technique in total arch repair for patients with type A dissection include: (I) the IA, LCCA and distal LSCA (at most 1 cm distal to its origin) are not involved by dissection, aneurysm; (II) the “island” is free from atherosclerotic and aneurysmal lesions; (III) adequacy of the circle of Willis (12).
Acceptable surgical results were obtained using this surgical procedure in the present study. The 5.7% in-hospital mortality was comparable to that of TAR using branched graft or classical en bloc technique. The SACP time was not significantly increased compared to conventional surgical procedure performed over the same period in our center (1), and no perioperative neurological deficits occurred. The short- and mid-term outcomes were favorable. Overall survival was 87.9% at 3 and 6 years, respectively. At 6 years, the incidence was 3% for reoperation, 12% for late death, and 85% of patients were alive without reoperation. The rate of false lumen thrombosis was comparable to previous FET series (2,4,12). Persistent anastomotic leak of the aortic arch was rarely observed (3.4%), which we think could be avoided by improving the suturing technique. Moreover, our technique has achieved excellent patency (100%) of the anastomosis between the LCCA and LSCA as confirmed by follow-up CTA.
Our modified en bloc technique has several advantages over the classical en bloc or branched graft technique. First, moving the distal anastomosis of the aortic arch to zone II (between the LCCA and LSCA) reduces technical complexity by simplifying anastomosis and hemostasis. Second, the small “island” is helpful in reducing the risk of aneurysmal dilatation. Third, different from artificial grafts that have the potential risk of stenosis or occlusion (29,30), all three native arch vessels are preserved by addition of the LSCA-LCCA transposition in this technique, which improves long-term patency. Fourth, only 3 anastomoses were needed during TAR. Furthermore, edema of the aortic wall diminished in most patients with CTAAD, making it firm enough to allow for strong traction of the sutures. Therefore, this surgical technique is more suitable for patients with chronic type A dissection with normal aortic tissues surrounding the IA and LCCA.
Study limitations
This study is limited by its retrospective nature, small sample size, lack of a control group and the relatively short duration of follow-up. Further studies in a large series, preferably in multiple centers, for longer durations are warranted to examine the long-term efficacy of this modified technique.
Conclusions
This modified en bloc technique with LSCA-LCCA transposition during TAR and FET procedure was safe and feasible and has achieve favorable mid- to long-term clinical and imaging results in patients with chronic type A dissection. It may be an alternate approach to chronic type A dissection in selected patients.
Acknowledgements
Funding: This study was supported by the National Science and Technology Support Program of China (No. 2015BAI12B03), Beijing Major Science and Technology Projects from Beijing Municipal Science and Technology Commission (Z171100001017083) and Beijing Lab for Cardiovascular Precision Medicine (PXM2017_014226_000037).
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board of Beijing Anzhen Hospital of Capital Medical University (No. 2016016X).
References
- Ma WG, Zheng J, Zhang W, et al. Frozen elephant trunk with total arch replacement for type A aortic dissections: Does acuity affect operative mortality? J Thorac Cardiovasc Surg 2014;148:963-70; discussion 970-2. [Crossref] [PubMed]
- Sun LZ, Qi RD, Chang Q, et al. Is total arch replacement combined with stented elephant trunk implantation justified for patients with chronic Stanford type A aortic dissection? J Thorac Cardiovasc Surg 2009;138:892-6. [Crossref] [PubMed]
- Di Eusanio M, Armaro A, Di Marco L, et al. Short- and midterm results after hybrid treatment of chronic aortic dissection with the frozen elephant trunk technique. Eur J Cardiothorac Surg 2011;40:875-80. [PubMed]
- Pacini D, Tsagakis K, Jakob H, et al. The frozen elephant trunk for the treatment of chronic dissection of the thoracic aorta: a multicenter experience. Ann Thorac Surg 2011;92:1663-70; discussion 1670.
- Hynes CF, Greenberg MD, Sarin S, et al. Chronic type A aortic dissection: two cases and a review of current management strategies. Aorta (Stamford) 2016;4:16-21. [PubMed]
- Katada Y, Kondo S, Tsuboi E, et al. Endovascular total arch repair using in situ fenestration for arch aneurysm and chronic type A dissection. Ann Thorac Surg 2016;101:625-30. [Crossref] [PubMed]
- Shrestha M, Martens A, Behrendt S, et al. Is the branched graft technique better than the en bloc technique for total aortic arch replacement? Eur J Cardiothorac Surg 2014;45:181-6; discussion 186-7. [Crossref] [PubMed]
- Di Eusanio M, Schepens MA, Morshuis WJ, et al. Separate grafts or en bloc anastomosis for arch vessels reimplantation to the aortic arch. Ann Thorac Surg 2004;77:2021-8. [Crossref] [PubMed]
- Chen LW, Dai XF, Lu L, et al. Extensive primary repair of the thoracic aorta in acute type a aortic dissection by means of ascending aorta replacement combined with open placement of triple-branched stent graft: early results. Circulation 2010;122:1373-8. [Crossref] [PubMed]
- Hirst AE Jr, Johns VJ Jr, Kime SW Jr. Dissecting aneurysm of the aorta: a review of 505 cases. Medicine (Baltimore) 1958;37:217-79. [Crossref] [PubMed]
- Sun L, Qi R, Zhu J, et al. Total arch replacement combined with stented elephant trunk implantation: a new "standard" therapy for type a dissection involving repair of the aortic arch? Circulation 2011;123:971-8. [Crossref] [PubMed]
- Zhu JM, Qi RD, Chen L, et al. Surgery for acute type A dissection using total arch replacement combined with stented elephant trunk implantation: Preservation of autologous brachiocephalic vessels. J Thorac Cardiovasc Surg 2015;150:101-5. [Crossref] [PubMed]
- Zhu JM, Qi RD, Chen L, et al. Stented elephant trunk procedure with left subclavian artery transposition for acute type B dissection with distal arch involvement. J Thorac Cardiovasc Surg 2015;150:1160-5. [Crossref] [PubMed]
- Peterss S, Mansour AM, Ross JA, et al. Changing pathology of the thoracic aorta from acute to chronic dissection: Literature review and insights. J Am Coll Cardiol 2016;68:1054-65. [Crossref] [PubMed]
- Carnevale D, Lembo G, Frati G. Chronic Type A aortic dissection: could surgical intervention be guided by molecular markers? J Cell Mol Med 2011;15:1615-9. [Crossref] [PubMed]
- Rylski B, Milewski RK, Bavaria JE, et al. Outcomes of surgery for chronic type A aortic dissection. Ann Thorac Surg 2015;99:88-93. [Crossref] [PubMed]
- Hanif H, Dubois L, Ouzounian M, et al. Aortic arch reconstructive surgery with conventional techniques vs frozen elephant trunk: a systematic review and meta-analysis. Can J Cardiol 2018;34:262-73. [Crossref] [PubMed]
- Takagi H, Umemoto T. A meta-analysis of total arch replacement with frozen elephant trunk in acute type A aortic dissection. Vasc Endovascular Surg 2016;50:33-46. [Crossref] [PubMed]
- Jakob H, Dohle D, Benedik J, et al. Long-term experience with the E-vita Open hybrid graft in complex thoracic aortic disease. Eur J Cardiothorac Surg 2017;51:329-38. [PubMed]
- Shimamura K, Kuratani T, Matsumiya G, et al. Hybrid endovascular aortic arch repair using branched endoprosthesis: the second-generation "branched" open stent-grafting technique. J Thorac Cardiovasc Surg 2009;138:46-52; discussion 52-3. [Crossref] [PubMed]
- Glauber M, Murzi M, Farneti P, et al. Aortic arch replacement with prophylactic aortic arch debranching during type A acute aortic dissection repair: initial experience with 23 patients. Eur J Cardiothorac Surg 2011;40:418-23. [PubMed]
- Antoniou GA, El SK, Hamady M, et al. Hybrid treatment of complex aortic arch disease with supra-aortic debranching and endovascular stent graft repair. Eur J Vasc Endovasc Surg 2010;39:683-90. [Crossref] [PubMed]
- Sun LZ, Ma WG, Zhu JM, et al. Sun's procedure for chronic type A aortic dissection: total arch replacement using a tetrafurcate graft with stented elephant trunk implantation. Ann Cardiothorac Surg 2013;2:665-6. [PubMed]
- Shen K, Tang H, Jing R, et al. Application of triple-branched stent graft for Stanford type A aortic dissection: potential risks. Eur J Cardiothorac Surg 2012;41:e12-7. [Crossref] [PubMed]
- Chang Q, Tian C, Wei Y, et al. Hybrid total arch repair without deep hypothermic circulatory arrest for acute type A aortic dissection (R1). J Thorac Cardiovasc Surg 2013;146:1393-8. [Crossref] [PubMed]
- Moulakakis KG, Mylonas SN, Markatis F, et al. A systematic review and meta-analysis of hybrid aortic arch replacement. Ann Cardiothorac Surg 2013;2:247-60. [PubMed]
- Spielvogel D, Strauch JT, Minanov OP, et al. Aortic arch replacement using a trifurcated graft and selective cerebral antegrade perfusion. Ann Thorac Surg 2002;74:S1810-4; discussion S1825-32.
- LeMaire SA, Price MD, Parenti JL, et al. Early outcomes after aortic arch replacement by using the Y-graft technique. Ann Thorac Surg 2011;91:700-7; discussion 707-8. [Crossref] [PubMed]
- Numata S, Tsutsumi Y, Monta O, et al. Mid-long-term results after aortic arch repair using a four-branched graft with antegrade selective cerebral perfusion. J Card Surg 2013;28:537-42. [Crossref] [PubMed]
- Kawasaki R, Sugimoto K, Taniguchi T, et al. Endovascular treatment for visceral vessel complication after branched graft replacement: initial results. AJR Am J Roentgenol 2008;191:1175-81. [Crossref] [PubMed]