Correlation between group behavior and quorum sensing in Pseudomonas aeruginosa isolated from patients with hospital-acquired pneumonia
Introduction
Pseudomonas aeruginosa (P. aeruginosa) is one of the most common opportunistic pathogens and a cause of serious hospital-acquired infection (1). This pathogen is persistent and easily forms biofilms, colonizing the body and causing outbreaks of cross-infections and other clinical manifestations. It is now well established that a variety of P. aeruginosa phenotypic features, including motility, virulence, and the ability to form biofilms are regulated by quorum-sensing (QS) systems (2,3).
A number of gram-negative bacteria, including P. aeruginosa, use acylated homoserine lactone (HSL)-based QS that in P. aeruginosa includes adjustable Las and Rhl signal systems. The Las signaling system comprises the LasR and LasI genes (4,5) encoding a transcriptional activator LasR and an enzyme LasI, which directs the synthesis of a signal molecule N-(3-oxododecanoyl)-
In this study, we assessed the expression of QS-related genes in P. aeruginosa clinical isolates from patients with acute lower respiratory tract infections and analyzed the relationship between the QS signaling systems and P. aeruginosa group behavior. We also compared these parameters in clinically controlled and persistent P. aeruginosa isolates with the aim of providing a basis for novel therapeutic strategies in the treatment of hospital-acquired P. aeruginosa infection.
Materials and methods
Patients and P. aeruginosa clinical isolates
This prospective study (ethics code: 2013) included patients treated from March to November 2010 at the respiratory general ward, emergency intensive care unit (EICU), respiratory intensive care unit (RICU), surgical intensive care unit (SICU), and cardiac surgical intensive care unit (CSICU) of the Shanghai Jiaotong University Affiliated Ruijin Hospital and at the respiratory general ward of the Ruijin Hospital Luwan Branch. Subjects (30 men and 18 women) were recruited among the patients with freshly diagnosed hospital-acquired pneumonia. All participants provided written informed consent. The clinical diagnostic criteria included chest radiography 48 h after the admission prompted by emerging or progressive exudative lesions combined with any two of the following three clinical manifestations: temperature above 38 °C, high blood leukocytosis, and purulent sputum. Patients with previously diagnosed P. aeruginosa infection were excluded from the study.
The analyzed clinical parameters included patients’ age, sex, disease complications, antibiotic treatment, and bacterial clearance at days 1, 7, and 14 after the treatment for P. aeruginosa infection. Secretions from lower respiratory tract via the endotracheal tube or after morning expectoration were collected in a mouthwash container filled with sterile saline. The isolates were streaked on LB agar and stored in 20% glycerol/LB broth at –80 °C.
The study endpoints included negative airway secretions, negative P. aeruginosa sputum culture within 14 days, or patient death.
Biofilm formation and quantification
P. aeruginosa isolates were grown overnight in LB medium at 37 °C. The cultures was subsequently diluted with tryptone broth (TB) to OD600 of approximately 0.02, and 10 µL of the diluted culture was added to 96-well flat-bottom tissue culture plates containing 200 µL of LB diluted 1:50. Each strain was added to six wells (blank control wells contained medium only), and the plates were incubated as static cultures at 37 °C for 48 h. Biofilms were washed with normal saline, dried at room temperature, and stained with crystal violet (0.1% in water, 150 µL/well) for 20 min at room temperature. The stained biofilms were washed three times with 1 mL of normal saline, and the dye was solubilized with 150 µL 95% ethanol and measured by absorbance at 570 nm (OD570) using a microplate reader (KHB ST-360, Shanghai, China). The biofilm formation rate (OD570)/(mg∙mL) was calculated as the average OD570 value of three measured wells minus the average of three blank wells.
Swimming motility assay
The flagellum-mediated motility of P. aeruginosa was assessed using plates containing 0.3% LB agar as a motility medium. A 1-µL aliquot of overnight LB cultures was inoculated in the agar, and after 16-h incubation at 37 °C, the diameter of the swim zone was measured. All assays were performed in triplicate (11).
Twitching motility assay
Plates containing 3-mm deep 1% LB agar were dried briefly, inoculated with P. aeruginosa isolates using a needle placed at the bottom of the plate, and incubated at 37 °C for 48 h (except when noted otherwise). After the incubation period, a zone between the agar and the plate bottom, referred to as the twitch zone, was measured. All assays were performed in triplicate (12).
Swarming motility assay
The isolates were tested for swimming motility on the plates containing 0.2% glucose, 0.05% monosodium glutamate, and 0.5% agar. A 1-µL aliquot of overnight LB cultures was placed on the agar surface, and the diameter of the swarm zone was measured after 48-h incubation at 37 °C. All assays were performed in triplicate (13).
Pyocyanin production assay
P. aeruginosa isolates were grown overnight in LB medium at 37 °C. The cultures were subsequently diluted with TB to OD600 of approximately 0.06, and 2.0 mL of the dilution was added to 24-well flat-bottom culture plates at 37 °C for 24 h. Cultures were extracted with 3 mL of chloroform and then reextracted into 1 mL of 0.2 N HCl to give a pink to deep red solution. The absorbance of this solution was measured at 520 nm. All assays were performed in triplicate (14).
RNA extraction and quantitative reverse transcription PCR (qRT-PCR)
Bacteria were grown in LB broth at 37 °C to the mid-exponential phase (OD600 1.0-1.4) for 24 h. Total RNA was isolated using the RNeasy Mini Kit (SBS, Takara, Kyoto, Japan) according to the manufacturer’s instructions. Differential gene expression was examined by real-time quantitative reverse transcription PCR (qRT-PCR) using the SYBR RT-PCR platform (Takara) according to the manufacturer’s instructions. Primer pairs were designed using the Primer Express software package (Takara):
LasI, sense 5'-GCCCCTACATGCTGAAGAACA-3', antisense 5'-GTCCAGAGTTGATGGCGAAA-3';
LasR, sense 5'-ACGCTCAAGTGGAAAATTGGA-3', antisense 5'-GGGTAGTTGCCGACGATGAA-3';
RhlI, sense p, 5'-AGCTTCTCGATGAAGACCTGATG-3', antisense 5'-TGCTCTCTGAATCGCTGGAA-3';
RhlR, sense 5'-TCGCTCCAGACCACCATTTC-3', antisense 5'-CCACACGATTCCCTTCACC-3'.
Prior to comparative analysis, the relative efficiency of each primer pair was tested and compared to that of the primer pair for ribosomal RplU (sense, 5'-TCGTGTCGGATGTTGGGTTA-3'; antisense, 5'-GGTTTCGCTGCCCTTTGTATTGT-3') to ensure that the threshold cycle (Ct) data analysis could be used. The absolute value of the slope of the log input amount versus the ΔCt was less than 0.1 for all comparisons, allowing us to use the ΔΔCt calculation to compare gene expression in the experimental cultures to that of the controls (15). The experiments were repeated at least three times.
Grouping according to bacterial clearance
The patients were grouped according to bacterial clearance after antibiotic treatments. The clearance was determined as the absence of P. aeruginosa in two consecutive lower respiratory tract specimens during the 14-day observation period. No-clearance was scored if P. aeruginosa was cultured from all lower respiratory tract specimens after the 14-day observation period.
Statistical analysis
The data were expressed as the mean ± standard deviation or as percentage (for invariable parameters). Correlation analysis was performed using the Spearman method; comparison between two groups was performed using the Kolmogorov-Smirnov Z rank-sum test. P values of <0.05 were considered statistically significant.
Results
General clinical data
The study population included 48 hospitalized patients, 30 men (62.5%) and 18 women (37.5%), with an average age of 68.18±15.08 years. There were 32 cases of ventilator-associated pneumonia (66.7%). After the antibiotic treatment, six cases with P. aeruginosa clearance (26.3%) and 42 cases without clearance (73.7%) were detected (Table 1). All 48 patients demonstrated hospital-acquired pneumonia complications, including 20 cases of neurological disease (41.7%), 17 of cardiovascular disease (35.4%), eight of lung disease (16.7%), seven of malignant kidney disease (14.6%), seven of diabetes (14.6%), five of acute pancreatitis (10.4%), and four of blood diseases (8.3%). Antibiotic therapy to clear P. aeruginosa infection included carbapenems (40 cases, 83.3%), β-lactam/β-lactamase inhibitors (15 cases, 31.3%), cephalosporins (9 cases, 18.8%), and fluoroquinolones (5 cases, 10.4%).

Full table
Relationship between QS gene expression and swimming motility
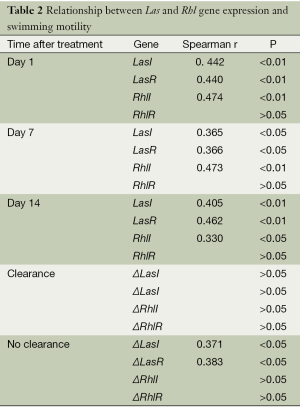
In total, 138 P. aeruginosa isolates were analyzed. At all time points (days 1, 7, and 14), P. aeruginosa clinical isolates demonstrated a significant positive correlation between swimming behavior and the expression of LasI, LasR (P<0.01 for days 1 and 14, P<0.05 for day 7), and RhlI genes (P<0.01 for days 1 and 7, P<0.05 for day 14). However, no correlation was observed between swimming motility and RhlR expression (P>0.05) (Table 2).
Full table
In the patient group demonstrating bacterial clearance after antibiotic treatment, the differences in the relative expression of LasI, LasR, RhlI, and RhlR before and after therapy (ΔLasI, ΔLasI, ΔRhlI, and ΔRhlR) were not correlated with the changes in swimming behavior (P>0.05). However, in the no-clearance group, the changes in swimming motility positively correlated with ΔLasI (r=0.371, P<0.05) and ΔLasR (r=0.383, P<0.05), whereas no correlation was observed for ΔRhlI and ΔRhlR (P>0.05).
Relationship between QS gene expression and twitching motility
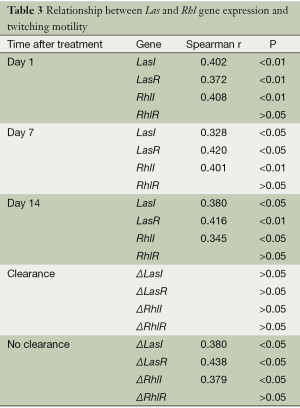
Similar to swimming behavior, twitching motility of P. aeruginosa clinical isolates at all time points showed a significant positive correlation with the relative expression of LasI (P<0.01 for day 1 and 14, P<0.05 for days 7 and 14), LasR (P<0.01 for days 1 and 14, P<0.05 for day 7), and RhlI (P<0.01 for days 1 and 7, P<0.05 for day 14), whereas no correlation was detected for RhlR expression (P>0.05) (Table 3).
Full table
In the patient group demonstrating bacterial clearance after antibiotic treatment, the changes in twitching motility did not correlate with ΔLasI, ΔLasI, ΔRhlI, or ΔRhlR (P>0.05), whereas in no-clearance group, they positively correlated with ΔLasI (r=0.380, P<0.05), ΔLasR (r=0.438, P<0.05), and ΔRhlI (r=0.379, P<0.05). However, no such correlation was observed between P. aeruginosa twitching and ΔRhlR (P>0.05).
Relationship between QS gene expression and swarming motility
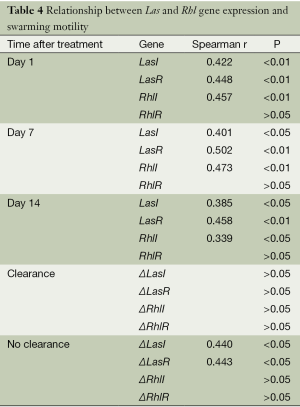
Swarming motility of P. aeruginosa clinical isolates at all time points showed a significant positive correlation with the relative expression of LasI (P<0.01 for day 1, P<0.05 for days 7 and 14), LasR (P<0.01), and RhlI (P<0.01 for days 1 and 7, P<0.05 for day 14), but not with that of RhlR (P>0.05) (Table 4).
Full table
Similar to the trend detected in other motility assays, in the patient group demonstrating infection clearance after antibiotic treatment, the differences in swarming motility did not correlate with ΔLasI, ΔLasI, ΔRhlI, or ΔRhlR (P>0.05); however, in the no-clearance group, they showed a significant positive association with ΔLasI (r=0.440, P<0.05) and ΔLasR (r=0.443, P<0.05). No such link was detected for the Rhl genes: ΔRhlI and ΔRhlR showed no correlation with swarming motility (P>0.05).
Relationship between QS gene expression and biofilm formation
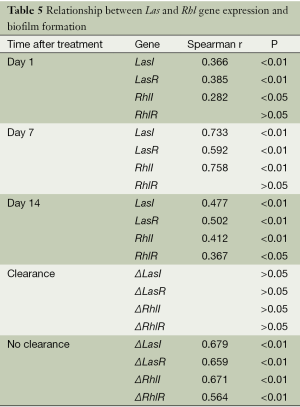
At all tested time points, a significant positive correlation was found between biofilm formation and the expression of LasI, LasR (P<0.01), and RhlI (P<0.05 for day 1, P<0.01 for days 7 and 14), whereas RhlR expression positively correlated with biofilm formation only on day 14 (P<0.05) (Table 5).
Full table
No correlation was observed between changes in biofilm formation and differential expression of QS genes in the patient group demonstrating bacterial clearance after antibiotic treatment (P>0.05). However, in patients with persistent P. aeruginosa infection (no-clearance group), a significant positive association was detected between altered biofilm formation and differential expression of Las and Rhl genes: ΔLasI (r=0.679, P<0.01), ΔLasR (r=0.659, P<0.01), ΔRhlI (r=0.671, P<0.01), and ΔRhlR (r=0.564, P<0.01).
Relationship between QS gene expression and pyocyanin production
P. aeruginosa clinical isolates from specimens collected on days 1 and 7 showed positive correlation between pyocyanin production and the expression of LasI and RhlI (P<0.05) but not with that of LasR and RhlR (P>0.05). For P. aeruginosa isolates from day 14, no correlation was observed between the pigment production and QS gene expression (P>0.05) (Table 6). For both clearance and no-clearance patient groups, the differences in the relative expression of the QS genes before and after antibiotic treatment did not correlate with changes in pyocyanin production (P>0.05).
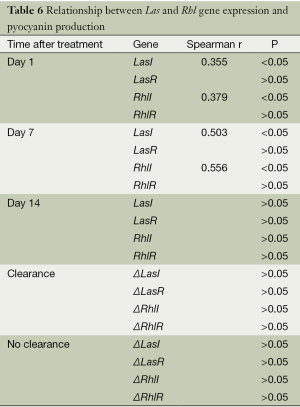
Full table
Comparison between patients with cleared and persistent P. aeruginosa infection
P. aeruginosa swimming motility was significantly different between the clearance (0.210±0.075) and no-clearance (–0.140±0.070) patient groups (P<0.05, Z=1.379). In contrast, there was no statistical difference between these patients in regard to P. aeruginosa twitching motility (0.060±0.028 and -0.060±0.033, respectively) and swarming motility (0.090±0.030 and -0.080±0.043, respectively) (P>0.05). However, a significant difference was observed between the clearance and no-clearance patients in P. aeruginosa biofilm formation (0.146±0.035 and 0.137±0.047, respectively) (P<0.01, Z=2.385) and pyocyanin production (0.064±0.044 and –0.066±0.028, respectively) (P<0.01, Z=1.938).
Discussion
In P. aeruginosa, quorum sensing systems play key roles in colonization and pathogenesis. An important phenotype regulated by QS is the communal movement of bacterial population. In this study of P. aeruginosa isolated from patients with hospital-acquired infection, we observed a strong positive correlation between the expression of QS genes LasI, LasR, and RhlI and all types of bacterial motility (swimming, twitching, and swarming), although no such correlation was detected for RhlR expression. These findings are consistent with previous data showing that P. aeruginosa QS Rhl genes regulated swimming, twitching, and swarming (16). Using genetic analysis, Caiazza et al. (17) showed that the regulation of P. aeruginosa swimming, twitching, and swarming motility depends on rhamnolipid biosynthesis controlled by the QS Rhl system (18). In P. aeruginosa, QS regulates the expression of several loci, including flagella and pili required for bacterial swarming movement, a phenotype important for community formation and host colonization.
In our study, a significant positive correlation was observed between P. aeruginosa biofilm formation and the expression of the Las signaling genes. However, a mixed pattern was detected for the Rhl system: RhlI was consistently associated with biofilm production, whereas for RhlR, such association was observed only on day 14. These data suggest that in P. aeruginosa virulent isolates, QS, especially the Las system, is critical for biofilm formation and thus for the bacterial parasitism of human hosts. Our data are in agreement with previous findings. O’Toole and Kolter (19) found that LasI mutation in P. aeruginosa resulted in a formation of defective flat, uniform undifferentiated biofilms lacking mature three-dimensional structure. Another study showed that in a P. aeruginosa RhlI (C4-HSL-deficient) mutant, the biofilm volume was reduced by 70%; the phenotype was rescued by the addition of exogenous C4-HSL, suggesting that the RhlI gene plays an important role in the formation of biofilms by P. aeruginosa (20,21). Xie et al. (22) observed that LasR and RhlR proteins induced biofilm formation by P. aeruginosa, indicating a direct involvement of the LasR and RhlR QC genes in the pathogen colonization of the host.
The secretion of P. aeruginosa virulence factor pyocyanin is under the control of the QS systems. In this study, we found that LasI and RhlI expression positively correlated with pyocyanin production on days 1 and 7, although no association was found on day 14. Another study suggested that LasR and RhlR mutations could be related to the spread of a drug-resistant strain of P. aeruginosa (23), although this mechanism requires additional investigation.
In the P. aeruginosa clearance patient group, no correlation was observed between pathogen communal behavior (motility, biofilm formation, and pyocyanin production) and the difference in the expression of the Las and Rhl signaling systems before and after antibiotic therapy. However, in the patients with persistent P. aeruginosa infection (no-clearance group), the differential expression of LasI, LasR, and RhlI was found to positively correlate with the changes in bacterial movement and biofilm formation, although no such link was detected for RhlR. Given that P. aeruginosa isolated from no-clearance patients exhibited increased biofilm formation, motility, and pyocyanin secretion compared to those isolated from clearance group, these results indicate that in the persistent clinical isolates, the Las and Rhl QS systems are directly associated with the pathogen communal behavior, suggesting QS involvement in P. aeruginosa drug resistance.
We believe that the investigation of the Las and Rhl signaling systems as potential targets in the control and treatment of P. aeruginosa infection represents a new research direction. In the present study, we collected samples from patients with hospital-acquired pneumonia at different time points and found that P. aeruginosa isolates reflected the patients’ conditions in terms of clinical development during treatment. Thus, we observed that the Las and Rhl genes of the QS systems were closely related to biofilm formation and other important parameters of P. aeruginosa group behavior, although previous data in this respect are controversial. Given that the current standard strains have been selected based on previous research, the results of this study may have a significant clinical impact.
However, this study also had several limitations. First, because of a small sample size, the results may not be representative of a larger population. In future investigations, we plan to expand the number of patients in order to increase the statistical power of association between the QS-related genes and P. aeruginosa communal behavior. In addition, mechanistic links between the QS genes should be addressed to better clarify their functions in vivo. Second, although we found the difference in QS gene expression between bacterial isolates from samples obtained before and after antibiotic treatment, we did not compare antibiotic type and dose and thus did not analyze specific factors influencing the regulation of QS genes. P. aeruginosa group behavior (biofilm formation, motility, and virulence factor secretion) is associated with clinically important chronic refractory infections. Therefore, the validation of the QS systems as potential drug targets for the control and treatment of P. aeruginosa infection requires further investigation.
Conclusions
In conclusion, our results indicate that the expression of QS genes, especially of the Las signaling system, in clinical isolates of P. aeruginosa is strongly associated with the pathogen communal behavior (motility, biofilm formation, and pyocyanin production) and resistance to antibiotic treatment, indicating the involvement of QS signaling in the clearance of P. aeruginosa infection.
Acknowledgements
Authors’ contribution: Jia-Lin Liu designed the study and performed the experiments. Yong Li performed the experiments, analyzed the data, and wrote the manuscript. Hong-Ping Qu and Huan-Ying Wan reviewed the data and participated in quality control. All authors read and approved the final version of the manuscript.
Funding: This work was supported by the National Natural Science Foundation of China (Grant No.81100004) and National Science and Technology Major Project (Grant No. 2011ZX09302-003-001).
Disclosure: The authors declare no conflict of interest.
References
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416. [PubMed]
- Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet 2001;35:439-68. [PubMed]
- González JE, Keshavan ND. Messing with bacterial quorum sensing. Microbiol Mol Biol Rev 2006;70:859-75. [PubMed]
- Williams P, Winzer K, Chan WC, et al. Look who’s talking: communication and quorum sensing in the bacterial world. Philos Trans R Soc Lond B Biol Sci 2007;362:1119-34. [PubMed]
- von Bodman SB, Willey JM, Diggle SP. Cell-cell communication in bacteria: united we stand. J Bacteriol 2008;190:4377-91. [PubMed]
- Pearson JP, Gray KM, Passador L, et al. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci U S A 1994;91:197-201. [PubMed]
- Latifi A, Winson MK, Foglino M, et al. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol Microbiol 1995;17:333-43. [PubMed]
- Whiteley M, Lee KM, Greenberg EP. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 1999;96:13904-9. [PubMed]
- Schuster M, Lostroh CP, Ogi T, et al. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol 2003;185:2066-79. [PubMed]
- Wagner VE, Bushnell D, Passador L, et al. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J Bacteriol 2003;185:2080-95. [PubMed]
- Schaber JA, Carty NL, McDonald NA, et al. Analysis of quorum sensing-deficient clinical isolates of Pseudomonas aeruginosa. J Med Microbiol 2004;53:841-53. [PubMed]
- Darzins A. The pilG gene product, required for Pseudomonas aeruginosa pilus production and twitching motility, is homologous to the enteric, single-domain response regulator CheY. J Bacteriol 1993;175:5934-44. [PubMed]
- Glessner A, Smith RS, Iglewski BH, et al. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of twitching motility. J Bacteriol 1999;181:1623-9. [PubMed]
- Essar DW, Eberly L, Hadero A, et al. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol 1990;172:884-900. [PubMed]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402-8. [PubMed]
- O’Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 1998;30:295-304. [PubMed]
- Caiazza NC, Shanks RM, O’Toole GA. Rhamnolipids modulate swarming motility patterns of Pseudomonas aeruginosa. J Bacteriol 2005;187:7351-61. [PubMed]
- Pamp SJ, Tolker-Nielsen T. Multiple roles of biosurfactants in structural biofilm development by Pseudomonas aeruginosa. J Bacteriol 2007;189:2531-9. [PubMed]
- O’Toole GA, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol 1998;28:449-61. [PubMed]
- Christensen LD, Moser C, Jensen PØ, et al. Impact of Pseudomonas aeruginosa quorum sensing on biofilm persistence in an in vivo intraperitoneal foreign-body infection model. Microbiology 2007;153:2312-20. [PubMed]
- Kirisits MJ, Parsek MR. Does Pseudomonas aeruginosa use intercellular signalling to build biofilm communities? Cell Microbiol 2006;8:1841-9. [PubMed]
- Xie Y, Zeng W, Jia WX, et al. Functional effects of LasR/RhlR on Pseudomonas aeruginosa biofilm development and lung infections in mice. Prog Biochem Biophys 2006;33:31-8.
- Fothergill JL, Panagea S, Hart CA, et al. Widespread pyocyanin over-production among isolates of a cystic fibrosis epidemic strain. BMC Microbiol 2007;7:45. [PubMed]


