Pulmonary and mediastinal paragangliomas: rare endothoracic malignancies with challenging diagnosis and treatment
Introduction
Tumors originating from chromaffin cells are located in 90% of cases in the adrenal gland and called pheochromocytomas, while in the remaining 10% of cases have an extra-adrenal origin (paraganglionic cells scattered throughout the body) and are termed paragangliomas (1,2).
Paragangliomas have been described at different sites: abdomen, pelvis, head, neck and thorax (1-4).
Endothoracic paragangliomas can arise in the lung (2,3,5) and in the mediastinum (originating from para-aortic and para-vertebral sympathetic chain ganglia) (1,6,7).
Because of their origin from neuroectodermal tissues (2), paragangliomas may have neuroendocrine activity, secreting catecholamines, similarly to pheochromocytomas (1). However, in the majority of cases paragangliomas are non-functional and the diagnosis is often incidental, in asymptomatic patients (up to 50% of cases) (1,6). When symptoms are present, apart from those related to catecholamine hypersecretion (hypertension, hyperhidrosis), a compression of surrounding organs (mass effect) may develop, causing dyspnea, dysphagia, hoarseness and chest pain (1,8).
Even if rare and most of them have a low grade of malignancy, endothoracic paragangliomas can present an aggressive behaviour, developing local infiltration of surrounding tissues and organs and distant metastases (2,9,10).
A correct pathological diagnosis and a radical surgical treatment, in case of localized forms, are fundamental to obtain clinical recovery (1,11).
We report our experience with three cases of endothoracic paragangliomas and a review of the literature, in order to point out difficulties in diagnosis and problems related to surgical treatment. This could be of help for clinicians and surgeons who do not know these rare tumors, in order firstly to consider them in the differential diagnosis and secondly to correctly manage them.
Methods
From January 2009 to December 2017, we observed and treated 3 patients (2 women, 1 man), mean age 67 years, with histological diagnosis of paraganglioma: 2 pulmonary, 1 mediastinal.
All patients were asymptomatic for catecholamine-secreting syndromes; the two cases with pulmonary localization showed no other symptoms, while the mediastinal one had episodes of cough and dyspnea, due to compression by the mass, and blepharospasm.
Imaging diagnosis was based on chest computerized tomography (CT) scan in pulmonary cases, chest CT and magnetic resonance imaging (MRI) scan for the mediastinal mass, which allowed to define vascularization and relationships with adjacent structures.
In one of the cases located into the lung, incidental chest CT diagnosis was made in a 67-year-old woman: a solid lung nodule of 3.7 cm in diameter was found in the right upper lobe, not associated to hilar and/or mediastinal lymphadenopathies. Bronchoscopy was negative; thus, the patient underwent right thoracotomy with diagnostic and therapeutic scope.
In the other case located into the lung, chest CT diagnosis was made in a 68-year old man during follow-up after right nephrectomy for renal carcinoma: a peripheral mass of 6 cm in diameter was found in the right lower lobe, associated with right hilar and mediastinal lymphadenopathies. PET-CT scan was positive both in the lung and right hilar and mediastinal lymph nodes (SUVmax 8.6 and 8.5 respectively). Bronchoscopic biopsies and endo-bronchial ultrasound trans-bronchial needle aspiration (EBUS-TBNA) of mediastinal lymph node station 4R and 7 were performed, but did not lead to pathological diagnosis (necrotic tissue), so the patient underwent right thoracotomy with diagnostic aim.
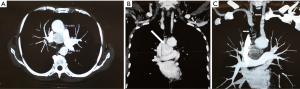
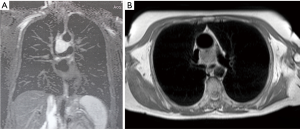
In the case with mediastinal localization, after neurological consultation and electromyography for bilateral ptosis, excluding the suspect of an ocular myasthenia and suggesting a blepharospasm, chest CT diagnosis was made in a 67-year old woman with aspecific respiratory symptoms (cough and episodes of dyspnea): a hypodense mass of 3.5 cm × 3.3 cm × 3.0 cm in diameter, with vascular enhancement, was found in the middle mediastinum, surrounded by the great vessels (superior vena cava, aortic arch, right pulmonary artery) but apparently showing cleavage from them; small vascular branches connecting the mass to the right subclavian and jugular veins and the descending aorta were also detected (Figure 1). Chest MRI confirmed the presence of a solid, highly vascularized lesion, with regular borders, in the middle mediastinum, posteriorly to the ascending aorta and above the right pulmonary artery (Figure 2). Therefore, the patient underwent right thoracotomy with diagnostic and eventually therapeutic objective.
No patient had preoperative histological diagnosis. Intraoperative pathological examination of the two pulmonary forms was suggestive for malignancy (extensive necrosis, high proliferative index, hypervascularization), thus a right upper lobectomy with hilar-mediastinal lymphadenectomy was performed in the female patient, a right pulmonary wedge resection with only diagnostic aim in the male one.
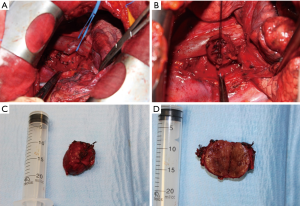
The hypervascularized mediastinal lesion, located in the middle mediastinum, tenaciously adherent to the superior vena cava, the aortic arch, the right branch of the pulmonary artery and the trachea, resulted positive for malignancy at intraoperative pathological examination and we were able to completely remove it after dissection, isolation and section of numerous vascular pedicles (Figure 3). Lymphadenectomy of station 2R and 4R was also performed.
Ethic approval was not required, as the manuscript report case series, nevertheless, informed consent was given by all the patients to use their data for scientific purposes.
Results
Postoperative course was uneventful in all cases.
Definitive histopathological examination revealed the diagnosis of paraganglioma (Table 1).

Full table
In the first pulmonary case, the tumor was capsulated, hypervascularized, composed of cords of epithelioid tumor cells with immunohistochemical positivity for neuron-specific enolase (NSE), synaptophysin and TTF1, negativity for chromogranin A (CgA) and cytokeratins (CKs) and sustentacular cells positive for S-100 protein; Ki-67 was 5% and the mitotic count was 2/10 high power fields (HPF). Surgical margins and lymph nodes were free from tumoral invasion.
In the second pulmonary case, the tumor showed a microscopic trabecular and nests pattern, extensive necrosis, globular cells with granular and eosinophilic cytoplasm, vesicular nucleus with nucleoli and frequent dysmetria, a rich microvasculature, immunohistochemical positivity for NSE, synaptophysin, chromogranin A, S-100 protein, vimentin, negativity for CKs, EMA, TTF1; the proliferative index was high (Ki-67 >40%) and the mitotic count was 25/10 HPF.
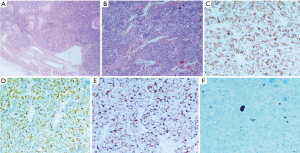
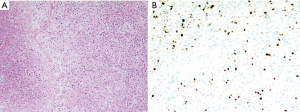
In the mediastinal case, the tumor was hypervascularized, with incomplete capsule, anaplastic nuclei, immunohistochemical positivity for chromogranin A, synaptophysin, CD56, negativity for CK 8/18, CK7, CK19, CD5, p63, thyroglobulin, calcitonin and a low proliferative index (Ki-67 10%); sustentacular cells were positive for S-100 protein; the mitotic count was 7/10 HPF (Figure 4). Capsular surgical margins and lymph nodes were free from tumoral invasion.
No patient received adjuvant treatments; periodical follow-up with CT scan was made.
At a median follow-up of 47 months (range, 6–102 months), two patients are alive, without local or distant recurrence; one male patient (with pulmonary involvement, extensive necrosis, high proliferative index and high mitotic count) died 6 months after surgery, due to disease progression (Figure 5).
Discussion
Paragangliomas are rare tumors (affecting 2 to 5 people per million per year) (10) with low grade of malignancy and non-epithelial origin, that are thought to arise from neural crest progenitor cells of the autonomic nervous system in extra-adrenal chromaffin tissues (1,2,6,12).
Endothoracic paragangliomas can grow in the lung or in the mediastinum. Primary pulmonary paragangliomas are extremely rare (<1% of all paragangliomas) (2) and can present as small multiple tumors or as a solitary mass (2,13); endobronchial involvement is very rarely reported (2). Generally, affected patients are females in their middle age (2).
Mediastinal paragangliomas represent less than 0.3% of mediastinal tumors and less than 2% of all paragangliomas (14,15) and are usually located in the bifurcation of great vessels, showing intense and homogeneous enhancement at chest CT scan (1).
Paragangliomas can be non-functional or functional, as they can secrete catecholamines (similarly to pheochromocytomas) (1,8). Patients with functional paragangliomas may develop hypertension, headache, palpitations, sweats and tremor (6). Primary pulmonary paragangliomas are usually non-functional and incidentally discovered as asymptomatic lung nodules suspected of being a primary lung cancer or a lung metastasis, as in our two cases (2,5,10). Sometimes aspecific respiratory symptoms, such as cough, chest pain and dyspnea, may be present.
Most of mediastinal paragangliomas are non-functional and incidentally detected at chest CT scan, as happened in our case; however, sometimes symptoms related to compression of adjacent mediastinal organs (heart, great vessels, trachea, esophagus) by the tumoral mass may develop, generally later in a patient’s life (16), and imaging is performed to clarify the clinical condition (1,6,8).
Paragangliomas of the middle mediastinum are generally non-functional and occur in older patients (as in our case), while those of the posterior mediastinum are usually functional and affect younger people (6,12,16).
However, functional middle mediastinum paragangliomas have been described (12,17), as well as non-functional posterior mediastinum ones (18) and even asymptomatic but functional paragangliomas of the posterior mediastinum (14,19).
Anterior mediastinum paragangliomas have also been reported, usually asymptomatic and non-functional (9); nevertheless, some Authors have reported that large size and anterior mediastinal location of these tumors are negative prognostic factors (20).
Macroscopically, paragangliomas are usually well-circumscribed, yellow-reddish in colour (hypervascular tumors) and hard in consistency, can be completely or incompletely capsulated and in this case may be firmly adherent to adjacent organs, thus making dissection and removal of the mass difficult, especially when located in the middle mediastinum (18).
Microscopically, they present typical anastomosing cords of tumor cells arranged in a trabecular pattern or a nesting pattern, separated by a rich microvasculature and this aspect can cause problems of differential diagnosis with carcinoid tumors. Paragangliomas are typically composed of two types of cells: chief cells and sustentacular ones. The chief cells are characteristically assembled in compact nests (“zellballen”), with clear or eosinophilic cytoplasm (containing neurosecretory granules) and rare mitoses, surrounded by sustentacular cells, which stain positively for S-100 protein (12). Immunohistochemical positivity for chromogranin A, synaptophysin and NSE is typically found in paragangliomas, while their negativity for cytokeratins and epithelial membrane antigens is an important differentiating factor from carcinoid tumors (12). However, paraganglioma’s chief cells can be of different types (epithelioid or ganglion-like cells), with different reactivity for chromogranin A (21); thus, some case can show positivity for synaptophysin, NSE and S-100 protein, but negativity to chromogranin A, as was in our first pulmonary case.
Preoperative diagnosis of endothoracic paragangliomas can be challenging and is based on clinical symptoms, imaging studies and urinary assay of catecholamine metabolites (1).
Generally, most of endothoracic, non-functional, pulmonary and mediastinal paragangliomas are incidentally detected at CT scan and the diagnosis is only confirmed postoperatively at pathological examination, as it was in our three patients. Some non-functional mediastinal forms can be suspected because of symptoms caused by the “mass effect” on surrounding organs (1,8).
In functional forms, a suspect of endothoracic paraganglioma can be suggested by typical symptoms caused by secretion of catecholamines: in case of hypertension (often paroxysmal) associated to the classic triad (headaches, palpitations and sweating), the specificity is reported to be more than 90% (14). These patients should undergo biochemical tests for the determination of the serum and urine levels of fractionated metanephrines, which is reported to be the most sensitive and specific examination for the diagnosis of such tumors (14,22,23).
Imaging is the cornerstone of diagnostics when suspecting endothoracic paragangliomas, as these tumors have typical features on CT and MRI scans (1): at CT, they present isodensity or slightly lower density and homogeneous, intense enhancement, except for necrotic areas with scarce enhancement; at MRI, they show intermediate signal intensity on T1-weighted images and high signal intensity on T2-weighted ones; moreover, mediastinal forms are usually located in the bifurcation of great vessels (1,7,10).
Thus, in addition to being non-invasive, CT and MRI not only allow to precisely define the characteristics and vascularity of the tumor, but also have the advantage of showing eventual tumor infiltration of the vascular and/or bronchial wall or lumen (16), which is a key point to be defined, in order to program a possible surgical resection. However, endobronchial paragangliomas detected at CT as hypervascular endobronchial mass can cause problems of differential diagnosis with endobronchial carcinoid or bronchogenic carcinoma (2).
In case of CT and/or MRI suspect of a paraganglioma, the mass can be better characterized by 123I-metaiodobenzylguanidine (123I-MIBG) scintigraphy (specificity between 95% and 100%, sensitivity of 90%) and 18F-fluorodeoxyglucose (18F-FDG) PET-CT scan (1,14,16,19).
In cases of mediastinal paraganglioma, additional information about cardiac and great vessels involvement can be obtained with transesophageal echocardiography (16).
Concerning non-invasive imaging diagnosis in our three patients, in one pulmonary case CT scan incidentally detected a solitary lung nodule, which was considered and treated as a bronchogenic carcinoma; in the other pulmonary case CT and PET-CT scan were performed during follow-up after nephrectomy for renal carcinoma and the suspect of lung metastases or primary lung cancer with right hilar and mediastinal lymphadenopathies was made; in the mediastinal case CT and MRI scan performed for aspecific symptoms allowed to detect a hypervascularized mass in the middle mediastinum.
Fibrobronchoscopy and EBUS can be used in the diagnostic protocol of endothoracic paragangliomas, in order to detect endobronchial mass and perform biopsies for pulmonary cases, and to study the characteristics of the hypervascularized mass for mediastinal ones; however, biopsies and TBNA during these endoscopic procedures, as well as CT-guided needle biopsy, can be hazardous, due to the proximity of the tumor to the great vessels and/or its intense vascularity (1,2,10). Moreover, diagnosis of paraganglioma based on bronchoscopic biopsies and/or needle biopsy only is difficult to achieve, because a complete pathological examination evaluating tumor morphology and structure as well as specific stains are necessary (1), especially for differential diagnosis from neuroendocrine tumors (5,10,12).
Regarding preoperative invasive diagnosis, in our first pulmonary case bronchoscopy resulted normal, while in the second pulmonary case bronchoscopy was positive, but endobronchial biopsies and EBUS-TBNA of station 4R and 7 yielded negative results, due to necrotic tissue; in the mediastinal case, EBUS-TBNA on the mass was not performed because of its intense vascularity and location adjacent to the great vessels. As none of our patients had preoperative diagnosis, surgical resection was programmed to achieve a definitive histological diagnosis.
Paragangliomas can have a spontaneous or genetic origin (16). Therefore, in the diagnostic protocol of all patients with paragangliomas, genetic testing should be considered, because 25–50% of these tumours are hereditary (6,14,16,24). Many Authors have studied genes associated with familiar paraganglioma: the most common mutations occur in the succinate dehydrogenase (SDH) complex of the electron transport chain, which is constituted by 4 proteins, labeled A to D (16,25,26). Mutations in the SDHB, SDHC and SDHD genes are clearly associated with an increased risk of paraganglioma (16,27-30). Paragangliomas can also be caused by mutations in other genes associated with particular familiar conditions, such as NF1 (neurofibromatosis type 1), VHL (von Hippel-Lindau disease), Ret (multiple endocrine neoplasia type 2), and PDGFRA (Carney triad, characterized by paragangliomas, gastric stromal tumors and pulmonary chondromas) (16,25,26,31).
Paragangliomas can show a tendency to local invasion and/or develop distant metastases, with a reported incidence of malignancy between 21% and 76% (2,9-11,18,22).
Therefore, complete surgical resection is considered the treatment of choice in functional and non-functional endothoracic paragangliomas (1,8-12,14,16-19,22,32,33); in fact, these tumors are relatively resistant to chemotherapy and radiotherapy (1,11,19,32).
In case of functional paragangliomas, preoperative alpha-adrenergic blockade should be administered, to reduce the risk of hypertensive crisis in the perioperative period; additionally, beta-adrenergic blockade and calcium channel blocker can be used for uncontrolled hypertension, even intraoperatively (8,17,18).
If diagnosis of functional paraganglioma is confirmed or strongly suspected, preoperative angiography is recommended, to study the vascular supply of the tumor and embolize it, in order to prevent intraoperative hypertension and reduce intra- and postoperative bleeding (17,18,22,34,35). In fact, the hypervascular nature of paragangliomas is well known, especially in those with mediastinal location, and haemorrhage during surgical resection may occur (8,18,19,35), even because these tumors are usually adjacent and adherent to mediastinal organs, such as the heart, great vessels, airways and spine, making complete removal challenging and difficult (1,8,9,11,18,19,22,32). Preoperative embolization can reduce the tumor vascularity and thus allow an easier and safe resection, without the need of more invasive procedures, such as cardiopulmonary bypass (22,35). On the other hand, some Authors advocate that embolization may induce, through tumor necrosis, catecholamines release and uncontrollable hypertensive crisis: therefore, they preferred to resect a mediastinal paraganglioma in sternotomy with cardiopulmonary bypass (17). Moreover, cardiopulmonary bypass may be necessary in anterior and middle paragangliomas strictly adherent or infiltrating the great vessels or the heart (8,11,18,36).
However, in most cases endothoracic paragangliomas can be resected by lateral thoracotomy (as was in our three patients) or sternotomy in a single-stage operation and intraoperative complications such as bleeding and hormonal-related crises can be prevented with a meticulous surgical technique and an accurate pre- and intraoperative blood pressure control (1,8,14,17,18).
As preoperative diagnosis is difficult, video-assisted thoracic surgery (VATS) could be the starting approach for mediastinal paragangliomas; however, the open one still remains the better approach in order to prevent and limit bleeding (18).
Nevertheless, some Authors have reported cases of successful complete resection of mediastinal paragangliomas using VATS, which allows an indirect manipulation of the tumor; however, a small size of the mass is the key-point to achieve a safe and complete VATS resection of these tumors (9,19).
After complete surgical resection, which provides the best long-term outcomes, prognosis of endothoracic paragangliomas is favourable (1,8,12). In a review about extra-adrenal chromaffin cells tumors Erickson et al. reported a surgical cure rate of 69% (37). Furthermore, over a follow-up period of 180 months in 79 cases with mediastinal paragangliomas, Lamy et al. reported an overall survival of 62% and in patients who received complete resection survival was 84.6% versus 50% (P<0.01) of those in whom resection was uncomplete (11).
The definitive diagnosis of paraganglioma can be achieved only with histopathologic examination, as was in our cases. Pericapsular invasion and the presence of a high number of mitosis are associated with a more aggressive behaviour, as was in one of our patients.
The role of adjuvant treatment for these tumors has not been yet established (9).
After surgery, semiannual and afterwards annual follow-up with imaging studies and biochemical testing, checking urinary catecholamines, especially in functional tumors, is recommended, to potentially detect recurrence and metastases (16).
Van Slycke et al. reported a mean incidence of recurrence of extra-adrenal paragangliomas of 15±7% at 5 years and 23±9% at 10 years after surgery (38). The reported incidence of malignancy for a pulmonary paraganglioma is approximately 18% (lower than that for paraganglioma in other sites) (2); however, there are reports of recurrence and metastases even after a long time: therefore, a life-long follow-up after surgical resection is mandatory (1,2,14,16).
Paragangliomas are usually low-grade indolent tumors, with an estimated probability of metastatisation ranging from 0% to 36%, depending on the type of tumor (39). The development of distant metastases is a negative predictor for long-term survival; the most common involved sites are lungs, lymph nodes and bones, suggesting both a lymphatic and hematogenous way of tumor spread (12,39).
However, as paragangliomas can arise at different body sites, can be multifocal, or can have a metachronous presentation, sometimes the diagnosis of metastatic disease can be difficult and complicated: a lesion should be considered as a metastasis only if present in a site where paragangliomas do not usually occur (12).
Mediastinal paragangliomas may develop a particularly aggressive behaviour and are associated with a higher morbidity and mortality (8,12,18), with distant metastasis rate reported as 19.5% and 26.6% in two review studies (11,40). Therefore, in limited disease and whenever possible, complete surgical resection should always be the treatment of choice. In almost 25 years’ experience of the Mayo Clinic on 14 mediastinal paraganglioma patients, a complete resection rate of 76.9% and a mortality rate of 7.1% were reported (8).
In the literature, a case of functional metastases of a non-functional paraganglioma has been reported, which was attributed to a phenotypic heterogeneity of the primary tumor, leading to a different biological behavior of its metastases (2,41).
In some cases of metastatic paragangliomas, successful treatment with sorafenib has been reported (10,42,43).
Conclusions
In conclusion, endothoracic paragangliomas, rare and often asymptomatic tumors, are of difficult diagnosis and even if most of them have a low grade of malignancy, should be considered malignant tumors, due to the potential aggressive behaviour of cases with high mitotic index and the frequent possibility of recurrence and metastases. Surgical resection is the treatment of choice and careful intraoperative manipulation is recommended, to prevent complications. After complete excision, long-term prognosis is generally good. However, even after surgical removal, a close, periodical and life-long follow-up is mandatory.
Acknowledgements
None.
Footnote
Conflicts of Interest: Presented on the 17th October 2017, as Poster, at the IASLC 18th World Conference on Lung Cancer, Yokohama, Japan, 15–18 October 2017.
Ethical Statement: Ethic approval was not required, as the manuscript report case series; nevertheless, informed consent was given by all the patients to use their data for scientific purposes.
References
- Wald O, Shapira OM, Murar A, et al. Paraganglioma of the mediastinum: challenges in diagnosis and surgical management. J Cardiothorac Surg 2010;5:19. [Crossref] [PubMed]
- Kim KN, Lee KN, Roh MS, et al. Pulmonary Paraganglioma Manifesting as an Endobronchial Mass. Korean J Radiol 2008;9:87-90. [Crossref] [PubMed]
- da Silva RA, Gross JL, Haddad FJ, et al. Primary pulmonary paraganglioma: case report and literature review. Clinics (Sao Paulo) 2006;61:83-6. [Crossref] [PubMed]
- Offergeld C, Brase C, Yaremchuk S, et al. Head and neck paragangliomas: clinical and molecular genetic classification. CLINICS 2012;67:19-28. [Crossref] [PubMed]
- Fiorentino G, Annunziata A, De Rosa N. Pulmonary paraganglioma of the lung:a case report. Journal of Medical Case Reports 2015;9:166. [Crossref] [PubMed]
- Young WF Jr. Paragangliomas: clinical overview. Ann N Y Acad Sci 2006;1073:21-9. [Crossref] [PubMed]
- Balcombe J, Torigian DA, Kim W, et al. Cross-sectional imaging of paragangliomas of the aortic body and other thoracic branchiomeric paraganglia. AJR Am J Roentgenol 2007;188:1054-8. [Crossref] [PubMed]
- Brown ML, Zayas GE, Abel MD, et al. Mediastinal paragangliomas: the Mayo clinic experience. Ann Thorac Surg 2008;86:946-51. [Crossref] [PubMed]
- Kim D, Kim SW, Hong JM. Mediastinal paraganglioma: complete resection using video-assisted thoracoscopic surgery. Korean J Thorac Cardiovasc Surg 2014;47:197-9. [Crossref] [PubMed]
- Huang X, Liang QL, Jiang L, et al. Primary Pulmonary Paraganglioma: A Case Report and Review of Literature. Medicine (Baltimore) 2015;94. [Crossref] [PubMed]
- Lamy AL, Fradet GJ, Luoma A, et al. Anterior and middle mediastinum paraganglioma: complete resection is the treatment of choice. Ann Thorac Surg 1994;57:249-52. [Crossref] [PubMed]
- Mehta CK, Gillespie CT, Lin X, et al. Rare Middle Mediastinal Paraganglioma Mimicking Metastatic Neuroendocrine Tumor. Ann Thorac Surg 2015;100:702-5. [Crossref] [PubMed]
- Saeki T, Akiba T, Joh K, et al. An extremely large solitary primary paraganglioma of the lung: report of a case. Surg Today 1999;29:1195-200. [Crossref] [PubMed]
- Parisinos CA, Carnochan FM, Gregoriades ML, et al. Mediastinal paraganglioma: time to panic? BMJ Case Rep 2011;2011.
- Ayadi-Kaddour A, Braham E, Ismail O, et al. Posterior mediastinal paragangliomas: a report of three patients with peculiar tumours. Respirology 2009;14:459-61. [Crossref] [PubMed]
- Ghouri MA, Krishnan E, Singh A, et al. Mediastinal Paraganglioma between the Great Vessels. Tex Heart Inst J 2013;40:189-92. [PubMed]
- Paul S, Jain SH, Gallegos RP, et al. Functional paraganglioma of the middle mediastinum. Ann Thorac Surg 2007;83:e14-6. [Crossref] [PubMed]
- Lin MW, Chang YL, Lee YC, et al. Non-functional paraganglioma of the posterior mediastinum. Interact Cardiovasc Thorac Surg 2009;9:540-2. [Crossref] [PubMed]
- Suzawa K, Yamamoto H, Ichimura K, et al. Asymptomatic but functional paraganglioma of the posterior mediastinum. Ann Thorac Surg 2014;97:1077-80. [Crossref] [PubMed]
- Ayala-Ramirez M, Feng L, Johnson MM, et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab 2011;96:717-25. [Crossref] [PubMed]
- Wang B, Wang B, Zou Y, et al. Duodenal gangliocytic paraganglioma:report of two cases and review of literature. Int J Clin Exp Pathol 2015;8:9752-9. [PubMed]
- Shakir M, Blossom G, Lippert J. Anterior mediastinal paraganglioma: A case for preoperative embolization. World J Surg Oncol 2012;10:134. [Crossref] [PubMed]
- Lenders JW, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is the best? JAMA 2002;287:1427-34. [Crossref] [PubMed]
- Neumann HP, Bausch B, McWhinney SR, et al. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med 2002;346:1459-66. [Crossref] [PubMed]
- Stratakis CA, Carney JA. The triad of paragangliomas, gastric stromal tumours and pulmonary chondromas (Carney triad), and the dyad of paragangliomas and gastric stromal sarcomas (Carney-Stratakis syndrome):molecular genetics and clinical implications. J Intern Med 2009;266:43-52. [Crossref] [PubMed]
- Matyakhina L, Bei TA, McWhinney SR, et al. Genetics of Carney triad: recurrent losses at chromosome 1 but lack of germline mutations in genes associated with paragangliomas and gastrointestinal stromal tumors. J Clin Endocrinol Metab 2007;92:2938-43. [Crossref] [PubMed]
- Baysal BE, Ferrell RE, Willett-Brozick JE, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 2000;287:848-51. [Crossref] [PubMed]
- Astuti D, Latif F, Dallol A, et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet 2001;69:49-54. [Crossref] [PubMed]
- Gimm O, Armanios M, Dziema H, et al. Somatic and occult germ-line mutations in SDHD, a mitochondrial complex II gene, in nonfamilial pheochromocytoma. Cancer Res 2000;60:6822-5. [PubMed]
- Baysal BE, Willett-Brozick JE, Lawrence EC, et al. Prevalence of SDHB, SDHC, and SDHD germline mutations in clinic patients with head and neck paragangliomas. J Med Genet 2002;39:178-83. [Crossref] [PubMed]
- Carney JA. Carney triad: a syndrome featuring paraganglionic, adrenocortical, and possibly other endocrine tumors. J Clin Endocrinol Metab 2009;94:3656-62. [Crossref] [PubMed]
- González-Santos JM, Arnáiz-García ME, Muñoz-Herrera A, et al. Mediastinal paraganglioma fed by the left circumflex artery. Interact Cardiovasc Thorac Surg 2016;23:835-6. [Crossref] [PubMed]
- Buchanan SN, Radeki KM, Chambers LW. Mediastinal paraganglioma. Ann Thorac Surg 2017;103:e413-4. [Crossref] [PubMed]
- Matsumoto J, Nakajima J, Takeuchi E, et al. Successful perioperative management of a middle mediastinal paraganglioma. J Thorac Cardiovasc Surg 2006;132:705-6. [Crossref] [PubMed]
- Rakovich G, Ferraro P, Therasse E, et al. Preoperative embolization in the management of a mediastinal paraganglioma. Ann Thorac Surg 2001;72:601-3. [Crossref] [PubMed]
- Andrade CF, Camargo SM, Zanchet M, et al. Nonfunctioning paraganglioma of the aortopulmonary window. Ann Thorac Surg 2003;75:1950-1. [Crossref] [PubMed]
- Erickson D, Kudva YC, Ebersold MJ, et al. Benign paragangliomas: clinical presentation and treatment outcomes in 236 patients. J Clin Endocrinol Metab 2001;86:5210-6. [Crossref] [PubMed]
- Van Slycke S, Caiazzo R, Pigny P, et al. Local-regional recurrence of sporadic or syndromic abdominal extra-adrenal paraganglioma: incidence, characteristics, and outcome. Surgery 2009;146:986-92. [Crossref] [PubMed]
- Fliedner SM, Lehnert H, Pacak K. Metastatic paraganglioma. Semin Oncol 2010;37:627-37. [Crossref] [PubMed]
- Olson JL, Salyer WR. Mediastinal paragangliomas (aortic body tumor). Report of 4 cases and a review of literature. Cancer 1978;41:2405-12. [Crossref] [PubMed]
- Fernandez-Llamazares J, Sabria-Leal M, Armengol-Carrasco M, et al. Functioning metastases of a nonfunctioning paraganglioma. J Surg Oncol 1988;37:213-4. [Crossref] [PubMed]
- Lin Y, Li Q, Huang W, et al. Successful treatment of paraganglioma with sorafenib: a case report and brief review of the literature. Onco Targets Ther 2013;6:1559-62. [PubMed]
- Gunaldi M, Kara IO, Duman BB, et al. A new approach to the treatment of metastatic paraganglioma: sorafenib. Cancer Res Treat 2014;46:411-4. [Crossref] [PubMed]


