Medical management of drug-sensitive active thoracic tuberculosis: the work-up, radiographic findings and treatment
Introduction
Tuberculosis (TB) is an ancient disease, believed to have afflicted human populations for millennia. Thought to have been vanquished by antimicrobial agents in the mid-20th century, TB has seen a resurgence in many parts of the world in the last few decades. A reservoir of 1.7 billion latently infected individuals (1) provides a source for increased incidence wherever exacerbating factors exist, including HIV/AIDS, prevalent drug resistance, or simply lack of public health infrastructure.
While largely a curable disease, TB ranks as the 9th leading cause of death worldwide and has now surpassed HIV/AIDS as the leading cause of death from a single infectious agent (2). Moreover, the mortality from TB pales in comparison to the tremendous morbidity even in those who are treated.
This review will summarize the most current literature on diagnostic workup, radiographic presentation and treatment for drug sensitive thoracic TB.
Diagnostic workup
A diagnosis of active pulmonary TB should be considered in all patients with suggestive clinical symptoms and epidemiologic risks such as a history of prior TB, known exposure, and travel to or residence in an endemic area (3). During a primary infection occurring when subjects are initially exposed to infectious bacilli, a fever may be the only presenting symptom (4). Many patients do not report localizing thoracic symptoms, with a minority having cough or chest discomfort. In reactivation (post-primary) pulmonary TB, patients usually present with an insidious onset of symptoms including cough, weight loss, fatigue along with fevers and night sweats. Most patients present for medical care only after being symptomatic for weeks to months. Because of a potentially long period after reactivation and the development of symptoms, it is not uncommon for TB screening programs to uncover asymptomatic active cases that may have been potentially infectious. Patients being evaluated for pulmonary TB who pose a public health risk with respect to transmission should be admitted and isolated with airborne precautions prior to a confirmed diagnosis.
For patients with suspected active thoracic TB, a number of different tests (summarized in Table 1) can be included in the workup. A tuberculin skin test (TST) or interferon-gamma release assay (IGRA) is routinely performed in some centers although it should be primarily used in the diagnosis of latent infection. A positive result supports (but does not confirm) a diagnosis of active TB disease, and a negative result does not rule out active TB disease (3,15) since up to 5% and 20% of patients with active disease may have a negative TST and IGRA, respectively. To more definitively establish a diagnosis of active pulmonary TB, M. tuberculosis should be isolated from a bodily secretion or tissue (16), but this has varying degrees of sensitivity and specificity depending on sample type, quality, extent of disease (e.g., cavitary disease) and host factors (e.g., immune status). Expectorated sputum examined with acid-fast bacilli (AFB) stain and culture is considered the first step in a workup for pulmonary TB. If patients are unable to provide a sputum sample via expectoration or induction, or if diagnostic samples remain negative, a bronchoscopy with bronchoalveolar lavage (BAL) or mediastinal lymph node aspirate can be performed. Sputum produced immediately after bronchoscopy should also be collected for AFB smear and culture to optimize diagnostic yield (3,17,18). Gastric aspirates can contain expectorated and then swallowed bacteria, and have been used diagnose TB in children who have difficulty providing a sputum specimen (14). In HIV-infected patients (with CD4 counts <100 cells/mm3), it is recommended to obtain mycobacterial cultures of blood and urine (in addition to the other studies) (15).
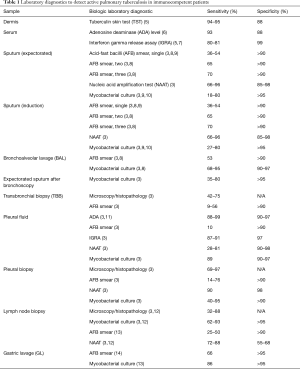
Full table
Depending on the region and facility where diagnostics are being run, several molecular methods may be available for detection of M. tuberculosis complex DNA. These modalities provide rapid pathogen detection and identification of mutations associated with drug resistance. Such molecular tests are now considered as part of a standard workup wherever accessible. While the details of these tests are beyond the scope of this review, the most common nucleic acid amplification tests (NAAT) in the United States are the amplified Mycobacterium tuberculosis direct (MTD) test and the Xpert MTB/RIF. The sensitivity and specificity of these tests vary based on specimen source, but they are generally considered more sensitive (>85% sensitive) than AFB smear in most studies. Moreover, the Xpert MTB/RIF allows for rapid rifampin (RIF) susceptibility testing but results should ultimately be verified by traditional culture-based susceptibility testing. Additional probe-based assays are available, along with investigational sequence-based assays which may hold the promise of resistance detection for a wider variety of medications.
In clinical cases with high suspicion of active pulmonary TB but without diagnostic confirmation, tissue biopsy may help establish a diagnosis, allowing both microbiologic studies and histopathologic examination. Pathology reveals granulomatous inflammation comprised of lymphocytes, epithelioid macrophages, and Langhans giant cells. Even in the absence of organisms on acid-fast staining, the characteristic appearance of caseation, or “cheese-like”, necrosis can help establish a diagnosis of active TB in the appropriate clinical and epidemiologic circumstances. However, this histologic appearance is not pathognomonic as other mycobacteria or fungal diseases can cause a similar appearance. Therefore, growth of the TB bacterium on culture is still required to establish a laboratory diagnosis.
Establishing a definitive laboratory diagnosis of TB may not be possible in some circumstances despite the many studies summarized in Table 1. In at least 15 to 20 percent of patients with a clinical diagnosis of active pulmonary TB, no confirmation is obtained despite laboratory bacteriologic testing (19,20). In such cases, a presumptive clinical diagnosis may be based on an epidemiologic exposure together with physical findings, radiographic findings, analysis of sputum or bronchoscopy specimens, and/or histopathology. In the setting of high clinical suspicion of TB without microbiologic confirmation, initiation of empiric therapy is appropriate as long as longitudinal reassessment for clinical and radiographic response is pursued.
Radiographic presentation
Clearly the workup of thoracic TB discussed above also relies heavily on radiographic imaging. The spectrum of radiographic presentation of thoracic TB can be highly variable depending on multiple factors including type of disease (primary versus post-primary), predominant location of infection (lymph node, parenchymal, pleural space), duration of infection and host factors such as age or immune status. Most practitioners are familiar with the radiographic presentation of reactivation (post primary) TB, but may be less so with the primary form. While it is convenient and common in the literature to discuss the radiographic presentation based on primary and post-primary classifications, in reality there is a continuum with significant overlap between the two (21). Furthermore, a distinction is less clinically relevant since active disease of either primary or reactivation is treated similarly. As with all diseases, the radiographic presentation is intimately tied to the pathogenesis and progression, which therefore deserves review.
Primary TB
Primary TB occurs when a host is initially exposed to mycobacterial bacilli via inhalation. Bacilli are carried by normal airflow largely to the mid and lower lung fields. An early granulomatous inflammatory response occurs known as a primary or Ghon focus. Because an effective cellular immune response can take weeks and vary from host to host, bacilli are initially able to grow and divide in the distal parenchyma before being carried to hilar lymph nodes via lymphatics and eventually more distant anatomic sites via the bloodstream. The ultimate outcome of a primary TB infection is dependent on the efficacy of this cell-mediated response and/or the initial inoculum. Most commonly, in ~90% of cases, the original and disseminated foci are walled off and destroyed (22). Primary or disseminated foci may also be walled off yet remain dormant and capable of reactivating in the future, causing post-primary disease. If the host immune status fails at initial containment, an unchecked primary infection (progressive primary TB) occurs with bacilli spreading hematogenously and endobronchially. Primary TB has been classically considered a disease of children living in highly endemic areas where exposure is more likely to occur during early years of life. However, the recognition of primary TB in adults in increasing (23,24) likely due to effective public health initiatives and treatment which have created larger populations of unexposed adults.
Based on the pathogenesis described above, it is not difficult to imagine why primary active TB is often characterized by one of the following:
- Hilar and mediastinal adenopathy are considered a “hallmark” of primary disease, seen in over 90% of children and 40% of adults (23). Even within adults, there exists an inverse relationship between age and radiographic lymphadenopathy, with young adults (<35 years old) more likely (43%) to have lymphadenopathy than older populations (10%) (4). Lymphadenopathy can be unilateral (preferentially right-sided) or bilateral, and on CT scan associated with a low attenuation center indicative of caseous necrosis. Adenopathy can be seen even in the absence of parenchymal disease, particularly in children. With appropriate treatment, lymph nodes typically decrease in size (Figure 1) but may paradoxically enlarge early in therapy (25), especially in the immunocompromised. This should not be interpreted as a treatment failure since it represents a heightened host immune response to bacterial degradation.
- Pulmonary infiltrates and consolidation occur in association with (and ipsilateral to) nodal enlargement in a large portion of children (~70%) (26) and adults (~80–90%) (27). Infiltrates occur more commonly in the middle and lower lung fields in adults, but in children upper and lower lobes are affected with equal frequency. While primary TB may involve the anterior segments of the upper lobes, post-primary TB usually involves other areas of the upper lobes. Infiltrates can range from very small, patchy and peripheral to more dense alveolar or interstitial shadows, and when present are indistinguishable from other pneumonic infiltrates. Cavitation within these infiltrates is unusual.
- Pleural effusion can often be the sole manifestation of primary TB and occurs when a parenchymal focus breaks through the visceral pleura. The effusion occurs either from direct infection of the pleural space or an associated delayed-type hypersensitivity reaction. Effusions increase in frequency with age, occurring in roughly 40% of adults with primary TB, but less commonly in children (27,28). They can be the only manifestation of primary disease, without pulmonary infiltrates or adenopathy in up to 5% of adults (4). Effusions are usually unilateral and of small to moderate size, but can be massive.
- If truly left unchecked, primary infection may progress to miliary (seed-like) TB occuring in 1–7% of cases (23,27). Milia appear as small, 2–3-mm nodules following a hematogenous distribution with slight lower lobe predominance (Figure 2). On CT scan, nodules are randomly distributed in relation to the secondary pulmonary lobule. They are initially present in the interstitium, but may coalesce radiographically to involve the airspaces.
- Volume loss/ atelectasis caused by lymph node airway compression or endobronchial lesions has been reported to occur in between 9–30% of children with primary TB but is less common in adults (29) because of the decreased prevalence of adenopathy and larger airways.
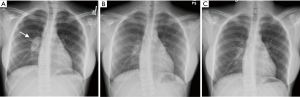
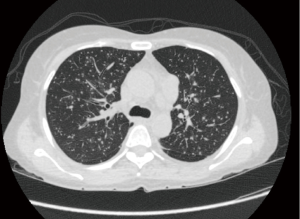
It is worthwhile to note that it is possible to have several or all of these different radiographic findings even in a single patient. Additionally, a normal chest X-ray (CXR) is possible in primary TB, with some series reporting up to 15% of cases (30), although this study did not include more sensitive CT scans to detect parenchymal lesions.
Post-primary (reactivation) TB
After being exposed to a primary inoculum of TB bacilli as discussed above, the immune system is usually able to control and/or eradicate the infection. However, in ~10% of patients, post-primary TB develops when a host is reinfected with TB or probably more commonly when a previously walled off primary focus begins to reactivate and proliferate. Some of the most important factors which could lead to reactivation are HIV, malignancy, organ transplant, chemotherapy, steroids, or TNF-alpha inhibitor medications.
The radiographic findings occurring in post-primary TB include:
- Upper lobe parenchymal infiltrates and consolidation with cavitation is the “hallmark” of post-primary TB (Figure 3). Infiltrates typically involve apical and posterior segments of the upper lobes, or the superior segment of the lower lobes. The predilection for the upper lobes is thought to be related to affinity of the bacilli for areas of high oxygen tension, and less efficient lymphatic drainage (31). Parenchymal infiltrates can appear as consolidation, a fibronodular pattern or both. Within areas of consolidation, single or multiple cavities occur in >50% of cases (28). Cavities classically have thick walls and may contain fluid as a result of caseous necrosis. Necrotic debris from these cavities may spread endobronchially to other sites, leading to multiple small nodules typically in the dependent lung zones. Fibronodular opacities without cavitation occur less frequently. Tuberculomas occur as a radiographic manifestation in ~5% and are solitary, sharply marginated nodules of 1–4 cm in diameter with associated adjacent satellite nodules (27,32).
- Volume loss/atelectasis in post-primary disease is caused by pulmonary fibrosis, unlike primary disease in which it is caused by lymph node airway compression or endobronchial disease. The fibrosis of post-primary TB is most common in the upper lobes, causing upward mediastinal retraction. Importantly, it is not possible by radiographic criteria alone to distinguish whether fibrosis represents active or inactive disease.
- Miliary disease in reactivation TB can result from dissemination of bacilli hematogenously, producing a similar pattern as described above.
- Pleural effusions can also form in reactivation TB, as in primary disease, via rupture of infectious foci or cavities into the pleural space. They can occur in 16–18% of post-primary cases (33) and are typically unilateral. The pleural space is more likely to be complicated in post-primary TB since a large number of organisms entering the pleural space from a ruptured cavity may lead to a bronchopleural fistula, loculated effusions, hydropneumothorax or frank tuberculous empyema.
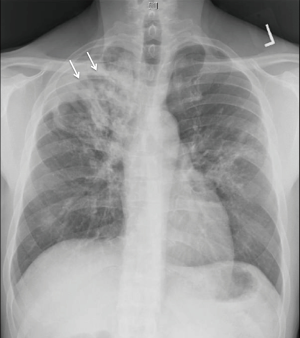
One key difference in reactivation TB is that mediastinal or hilar adenopathy is uncommon, occurring in only 5–10% of cases (27). However, similar to primary disease, several or all of these different radiographic findings can occur in a single patient (Figure 4). The spectrum of radiographic presentation common in both primary and post-primary active pulmonary TB is summarized graphically in Figure 5.

Chest radiograph of a 54-year-old man presenting with cough for 4 months and 50-pound weight loss. There is a dense right upper lobe infiltrate with areas of cavitation (arrows) indicative of post-primary TB. He was AFB smear positive and culture positive for pan-sensitive bacilli. TB, tuberculosis; AFB, acid fast bacilli.
Post-primary active TB presenting with multiple concomitant radiographic findings in a 37-year-old man with active disease 20 years prior but incomplete therapy due to medication cost. Radiograph reveals multiple calcified nodules (solid arrows) and upper lobe fibrotic areas (*) typically seen after healed TB, but also the presence of a pleural effusion (x) and parenchymal infiltrates (open arrows), some of which were cavitary on CT. The patient was smear positive for AFB, grew pan-sensitive MTB, and the pleural effusion had an elevated adenosine deaminase but was sterile. As stated in the text and demonstrated by this case, imaging frequently cannot distinguish active and inactive disease. TB, tuberculosis; AFB, acid fast bacilli; MTB, Mycobacterium tuberculosis.
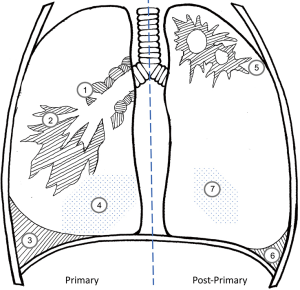
A summary of the typical radiographic manifestations in primary (left panel) and post-primary tuberculosis (right panel). Primary disease is largely characterized by mediastinal or hilar adenopathy which may cause airway compression (“1”) along with middle and lower lobe infiltrates (“2”) in adults. When unchecked, progressive primary disease leads to miliary spread (“4”). Pleural effusions (“3”) can be the sole manifestation of primary disease. In contrast, the parenchymal infiltrates of post-primary TB are usually apical and cavitary (“5”) with pleural effusions (“6”) and miliary spread (“7”) less common. Fibrotic changes may lead to volume loss with hilar or fissure displacement. Significant mediastinal adenopathy is uncommon in post-primary disease. While it is possible for several or all of these different radiographic findings to occur simultaneously in a single patient, this is less common and drawn in this manner for educational purposes.
Immunocompromised
When TB occurs in individuals with abnormal immune systems, the course of disease and radiographic presentation may vary. In individuals who have an immunocompromising condition but are still able to mount a response, the manifestations of TB most commonly parallel typical post-primary findings, including upper lobe cavitary disease. However, as the degree of immunosuppression increases, the radiographic presentation more closely mirrors primary disease. This was demonstrated in a prospective multicenter study including 128 HIV positive patients which compared X-ray findings to CD4 T-lymphocyte counts (34). The authors found that those patients with CD4 counts <200 cells/µL were more likely to appear radiographically as primary disease with mediastinal/hilar adenopathy and without cavitation. Progression to miliary disease is also associated with severe immunosuppression.
Post TB sequelae
After a primary TB exposure, the most common outcome is containment by an effective cell-mediated and delayed-type hypersensitivity host immune response. Primary TB usually resolves, forming a nodular opacity at the site of the Ghon focus along with calcified hilar or mediastinal lymph nodes. Most cases heal with minimal fibrosis and atelectasis. In contrast, after effective treatment or immune response in post-primary TB infection, fibrosis and scarring associated with volume loss occur, largely correlating to the original burden of parenchymal disease. Imaging cannot reliably distinguish active and inactive disease. In fact, viable TB bacilli may be grown from calcified lesions collected at autopsy (35). Additionally, bronchiectasis can occur as a result of damaged airways leading to hemoptysis or abnormal airway clearance. Residual cavities can be seen in up to 20% of patients (36) and can also predispose patients to aspergillomas. Finally, broncholithiasis can occurs years after TB infection, resulting in erosion of peribronchial lymph nodes into the airway causing hemoptysis, obstruction, or recurrent pneumonia. Many of these sequelae discussed above can be avoided with prompt diagnosis and thorough treatment.
Treatment
The decision to initiate treatment for active TB incorporates multiple forms of evidence for disease including clinical symptoms, microbiologic specimens (AFB smear, molecular tests, mycobacterial culture, histological/pathological findings), and imaging findings. In situations of uncertainty, the decision to treat may be driven also by a high index of suspicion and public health concerns for potential transmission. Clinicians should not wait for proof from microbiological specimens to start patients on TB therapy in such cases, especially if the patient is seriously ill. Prompt treatment aims to decrease bacillary burden, which not only lowers disease severity for the patient, but also limits infectivity (37,38). These goals are best achieved with a regimen of multiple drugs which ideally leads to swift bacterial killing and helps minimize drug toxicity, which in turn raises the likelihood of treatment completion. Moreover, anti-tuberculous agents target not only actively replicating bacteria but also more dormant “persisting bacteria”, thereby helping to achieve a durable cure and prevent relapse. Finally, the inclusion of several drugs prevents the development of drug resistance. In this way, treatment regimens address both individual and population health.
Public health and case management
Given the need for treatment with several drugs over a duration of months, a robust public health infrastructure is often crucial for achieving completion of therapy. The primary responsibility for successful treatment or cure should be placed on the health system and not on the patient. A case manager should develop an individualized care management plan based on patient needs and challenges, helping to coordinate a smooth transition from the hospital to continued therapy in the community. The desired goal is patient-centered care in which the patient, as an informed treatment ally, participates in decisions aimed at finding the best approach to successful completion. Systematic review has shown that directly observed therapy (DOT) by a provider yields superior results for some outcome measures over self-administered therapy (SAT). Therefore, DOT remains officially recommended and the standard of practice in the United States and many countries. However, some patients find DOT stigmatizing and increasingly, video DOT (VOT) via smartphones is being used as a more feasible alternative to traditional DOT. In rare cases, legal confinement is a last resort to ensure treatment completion.
Standard medication selection
Decades of research including multiple clinical trials (39) has settled on the following standard regimen for the treatment of drug-susceptible TB with some important caveats (discussed below): a 2-month intensive phase of 4 drugs including isoniazid (INH), RIF, pyrazinamide (PZA), and ethambutol (EMB) (abbreviated RIPE or HRZE) followed by a 4-month continuation phase of 2 drugs (INH and RIF). Whereas INH achieves significant early bactericidal activity (EBA), RIF targets “persisting” bacteria to achieve a “sterilizing effect” that enables relapse-free cure. PZA also has sterilizing activity but likely only in compartments of increased acidity. EMB primarily helps to prevent the development of resistance, especially given the increasing rates of INH resistance in many regions of the world. If sensitivity can be confirmed to INH and RIF, EMB may be discontinued and the patient may complete the intensive phase on 3 drugs (INH, RIF, PZA). A number of fixed-dose combinations (FDCs) are available to decrease pill burden, achieving higher patient satisfaction rates without compromising effectiveness. For these reasons, the WHO recommends use of FDCs over separate drug formulations when the former is not contraindicated by the need for specially tailored regimens or dosing changes (e.g., for renal or hepatic failure). Certain conditions (malnutrition, advanced age, pregnancy, breastfeeding in infants, HIV, diabetes mellitus, alcoholism, chronic renal failure) confer a risk of INH-induced neuropathy and these patients should also receive 25–50 mg/day of pyridoxine (vitamin B6). If neuropathy develops despite this, the dose is increased to 100 mg/day. As discussed below, it is important to obtain baseline liver function tests and ophthalmologic evaluation (for EMB) before starting treatment.
An important factor to consider when starting TB drugs is the effect on other medications the patient may be taking. RIF is one of most potent inducers of P450 (CYP) enzymes while INH is an inhibitor of several CYP enzymes. Whereas RIF may lead to enhanced metabolism of many drugs, INH may lead to toxicity from high concentrations of drugs such as anticonvulsants, benzodiazepines, warfarin, and acetaminophen. It is therefore our practice to perform a drug interaction review on all patients commencing therapy and make any necessary dosing adjustments to both the TB and coexisting drugs. Furthermore, when TB medications are discontinued, the dosing of interacting drugs should be adjusted accordingly.
Dosing frequency and duration
Daily dosing of TB medications is considered standard in 2018. Previous guidelines gave cautious recommendations for intermittent dosing following 2–3 initial weeks of daily therapy in those with difficulties adhering to daily dosing (40). However, the 2017 WHO Update no longer recommends intermittent dosing for any part of the regimen after incorporating data from several recent randomized controlled trials (41). Thrice-weekly dosing conferred increased risks of treatment failure, disease relapse, and acquired drug resistance. Therefore, intermittent dosing should never be used in the intensive phase and only as an inferior alternative in the continuation phase if effective monitoring is possible. Twice-weekly or once-weekly dosing [including with rifapentine (RPT) in place of RIF] should not be used for active disease. Intermittent dosing has never been advised for patients with cavitary disease or HIV co-infection. On the other hand, several studies have suggested clinical equivalency for DOT 5 vs. 7 days a week (i.e., daily therapy), although these two strategies have yet to be compared in a clinical trial (40).
While 6 months is the usual duration of treatment, an extended course is recommended for particular patients with a higher risk of relapse after 6 months of therapy. Such patients include those with cavitation on initial imaging, and those with persistently positive cultures at 2 months. Meeting both of these criteria resulted in a relapse rate of 20% vs. 2% in those with neither (42). Therefore, experts extend treatment for an additional 3 months of the continuation phase (i.e., 7 months of continuation phase and 9 months total). Extending treatment in this manner is also commonly done for patients with comorbidities including weight >10% below ideal body weight, active smoking, diabetes, HIV or any other immunosuppressing condition, and extensive disease on imaging.
Following the initiation of 4-drug therapy, sputum samples should be collected at least monthly (if necessary with the aid of induction) until 2 consecutive samples are culture negative. If the initial smear was positive, repeat sputum samples may be collected every 2 weeks to better gauge the response to treatment, particularly if there are heightened concerns for transmission to others (e.g., having a higher burden of AFB in the sputum). For those with cultures that remain positive even from sputa collected after 3 months of therapy, drug sensitivity testing (DST) should be repeated to screen for the development of resistance (i.e., “acquired” resistance). It is important to note that when collecting serial specimens throughout treatment, a positive smear or molecular test in the absence of culture growth can represent non-viable bacilli and not necessarily indicate treatment failure. Moreover, molecular testing should not be used to monitor response to therapy, given its high sensitivity but poor specificity compared with the reference of combined smear and culture results (43).
Alternative regimens
If INH or EMB cannot be used in the initial regimen due to significant intolerance or strict contraindications, a fluoroquinolone (FQ) (moxifloxacin or levofloxacin) may be substituted, though as of yet there are no clinical trials to support this action. Use of a FQ does not mean that the treatment course can be shortened, as 4-month regimens with FQ substitutions were shown to be less effective than the standard 6-month regimen and exhibited no reduction in adverse events or mortality (44,45). While 4-month FQ-based regimens had a slightly higher (though not statistically significant) rate of culture conversion at 2 months, there were higher rates of relapse at 18 months. A FQ may replace INH or EMB, but it cannot replace RIF or PZA. If PZA cannot be included (e.g., due to hypersensitivity, gout, or liver damage), experts advise an extended course consisting of a 2-month intensive phase of INH, RIF, and EMB, followed by 7 months of INH and RIF. As a potent CYP inducer, use of RIF may be contraindicated with certain medications such as highly active antiretroviral therapy (HAART) or immunosuppressive agents like cyclosporine. However, in some cases rifabutin (RFB), a less potent inducer, could instead be considered (46). If RIF or multiple first-line agents cannot be used in cases of drug-sensitive TB, then drug-resistant regimens consisting of second-line agents are employed. However, given the significant superiority in effectiveness of first-line agents, efforts should be made to preserve regimens containing INH, RIF, and PZA.
Trials are underway to investigate the repurposing of existing drugs (e.g., linezolid, carbapenems), higher dosing of current TB drugs, or the use of new agents for drug-resistant TB (bedaquiline, delamanid, pretomanid) in regimens for drug-sensitive TB as well. Moreover, a main goal of future therapy is to safely shorten the treatment course from 6 months, which has yet to be achieved. Imaging modalities including positron emission tomography (PET) have been used to monitor treatment response and hold some promise for allowing a shortening of therapy in particular individuals. A 4-month regimen may currently be contemplated for culture-negative TB but this remains controversial (see below).
Poor response or treatment failure, and retreatment
First-line TB drugs are highly effective even for patients with extensive lung damage. Nevertheless, some patients will have difficulty clearing cultures despite appropriately selected chemotherapy and a subset of these will remain symptomatic. The WHO defines treatment failure as having a positive sputum smear or culture at month 5 or later during treatment (47) (month 4 in the U.S.). There are several possible mechanisms for treatment failure. Health systems may fail to reliably deliver medications and nonadherence may occur even with DOT. Malabsorption or drug interactions may lower serum drug concentrations, which can be measured by therapeutic drug monitoring (TDM) (48). A failing regimen should raise suspicion for drug resistance, particularly if initial sensitivity was not obtained. If the patient is gravely ill, empiric therapy for drug-resistant TB may be started immediately, otherwise providers may opt to wait for repeat DST results in more stable patients. A single drug should never be added to a failing regimen as this risks the development of resistance to that new drug. For this reason, the WHO no longer recommends the retreatment category II regimen, which added streptomycin to RIPE (41). For patients previously treated for TB who present with the disease for a second time, the risk of drug-resistant TB is higher—especially if a rifamycin was not initially used, and DST should always be employed to target therapy. If immediate empiric therapy is necessary, the use an expanded regimen (i.e., RIPE plus a later-generation FQ, an injectable, and another second-line drug) under DOT and in consultation with an expert may be considered (40). Molecular tests should be used to more quickly detect potential resistance, though false positives have been reported (49).
It is important to note that an initial exacerbation of symptoms or transient radiographic worsening (as mentioned above) on appropriate TB therapy is common and should not be mistaken for treatment failure. However, such “paradoxical reactions” are diagnoses of exclusion and other possible etiologies should be considered.
Therapy interruptions
Interruptions in treatment commonly occur due to side effects or simply non-adherence. The clinical significance of an interruption is dependent on its duration, and when in the treatment course it occurs. Interruptions during the intensive phase (particularly early on) are more problematic since a higher number of actively replicating organisms are present and the risk of developing drug resistance is therefore higher. Accordingly, TB treatment programs have suggested requirements, which if not met, trigger a restart of the regimen. In one such scheme, if the break in therapy is 14 days or greater during the intensive phase, or if the break is less than 14 days but the total number of intensive phase doses cannot be completed within 3 months, then the regimen is restarted. Similar stipulations may be set for the continuation phase. In general, the total number of doses for a 6-month course should be given within 9 months, otherwise the regimen should be restarted (40). Treatment interruptions should trigger repeat sputum cultures. If these are positive, DST should be performed, and the regimen may need to be extended. For those lost to follow-up, if sputum cultures are negative at restart, they may be treated like patients with culture-negative TB (see below).
Side effects
While adverse effects of drug therapy are common, drugs should only be stopped if the side effects are severe; otherwise patients may be treated symptomatically. Short interruptions of drug therapy are common (as above), but for those with severe TB, an alternative regimen should be given while standard therapy is held. In these instances, expert medical consultation is advised, often with the aid of local and state health departments. Patients may be re-challenged with first-line agents. However, if permanent discontinuation of a particular drug is necessary, it should be replaced with an alternate agent preferably from a separate drug class.
The most common side effects are gastrointestinal and often occur early in therapy. Symptoms such as nausea, vomiting, and epigastric pain can develop with or without hepatotoxicity. For those symptomatic but lacking hepatotoxicity, strategies include bedtime dosing and antacid administration. With the exception of RPT, the bioavailability of which increases with high-fat meals, first-line TB drugs should be taken on an empty stomach for maximum absorption. Antacids are less likely to impact the absorption or peak concentrations of these drugs than taking them with food. Up to 20% of patients on the standard regimen will demonstrate an asymptomatic increase in ALT, which should provoke more frequent clinical and laboratory monitoring. Rises in Tbili and Alk Phos disproportionate to AST/ALT may herald RIF hepatotoxicity.
Drug-induced liver injury (DILI), or hepatotoxicity, is the most frequent serious complication of first-line drugs and may be caused by INH, RIF, PZA, or some combination thereof. Baseline liver function tests should be obtained prior to starting a standard regimen. Patients who use alcohol or have chronic viral hepatitis are especially at risk. If the ALT rises to 3 times the upper limit of normal (ULN) or greater in the presence of hepatitis symptoms (or 5 times the ULN without hepatitis symptoms), then DILI is suspected, and hepatotoxic drugs should be stopped immediately. It is important to rule out alternative causes of liver injury including viruses, biliary tract disease, and other hepatotoxic medications, supplements, or substances (e.g., acetaminophen, alcohol). When symptoms have resolved, and laboratory values have returned to baseline levels (or <3 times the ULN), agents are added back sequentially in a “re-challenge”. RIF should be restarted first, given that it causes less hepatotoxicity than INH or PZA. If there is no ALT increase after several days to a week, INH may be added and so on. The optimal re-challenge protocol remains unclear. If hepatitis was severe and INH and RIF are tolerated on re-challenge, PZA is assumed to have been the culprit and is discontinued. In these instances, therapy is often extended by 3 months, particularly if relatively few PZA doses have been given. If a week or more has elapsed and transaminases are still greater than 3 times the ULN, a “liver-sparing” regimen including second-line agents may be started until a re-challenge is possible. The official American Thoracic Society (ATS) statement may be consulted for further information on hepatotoxicity from TB drugs (50). If reintroduction of a full regimen occurs over weeks, it is reasonable to extend the overall course of therapy.
Rash may occur with any anti-tuberculous drug. Management depends on severity and ranges from continuation of medicines with the addition of antihistamines to cessation of medications for serious conditions like Stevens-Johnson Syndrome, or drug hypersensitivity syndrome. Petechiae in conjunction with thrombocytopenia are concerning for hypersensitivity to a rifamycin drug, in which case it should be permanently stopped. When other severe reactions occur, it may be unclear which drug is the offending agent. Upon resolution of the reaction, close observation is required for sequential re-introduction of single drugs. Corticosteroids have been used to manage severe systemic reactions without worsening TB outcomes.
About 2.25% of patients given standard doses of EMB will develop visual disturbances indicative of optic neuritis and therefore all patients should have a baseline eye exam at the start of therapy. Symptoms usually manifest after a month of therapy but can occur within days of starting EMB. During the period of EMB exposure, monthly tests of visual acuity (Snellen test) and color discrimination should be performed, and the drug promptly stopped if abnormal. Peripheral neuropathy from INH was addressed above.
HIV-positive patients
Patients with HIV should generally receive a standard regimen for TB treatment. The only major exception involves drug-drug interactions between RIF and antiretrovirals (ARVs). In some instances, RIF may be retained with dose adjustments to ARVs. In other cases, RIF is contraindicated, though the less potent CYP inducer RFB may be used in its place (46). Patients with HIV should only receive daily dosing of TB drugs, since intermittent dosing has been shown to result not only in increased relapse rates, but also the development of resistance to rifamycins. Older studies suggested extending the duration of therapy in HIV-positive individuals but included methodological flaws. Therefore, individuals with HIV and TB are generally treated with 6 months of therapy (as for HIV-negative individuals) with the exception of those not yet on HAART, in which case a 9-month course is advised. For those recently diagnosed with both TB and HIV, TB therapy is started first, with the timing of HAART initiation determined by the patient’s CD4 count (within 2 weeks if CD4 <50 copies/mL, within 8 weeks if CD4 ≥50 copies/mL) (51,52). Such a scheme avoids withholding HAART for too long in those who benefit from it the most, while attempting to decrease the risk of developing the immune reconstitution inflammatory syndrome (IRIS). If IRIS develops, it is rarely fatal. In general, it may be treated with glucocorticoids or other anti-inflammatories while continuing HAART and TB therapy.
Culture-negative pulmonary TB in adults
Despite suggestive history, symptoms, and/or radiographic findings, a subset of patients will not show evidence of Mycobacterium tuberculosis on sputum smear or culture. While alternative diagnoses are possible and should be pursued, many individuals may still have TB. Negative cultures may be explained by recent exposure to antibiotics with activity against TB (e.g., FQs), a low bacillary burden (i.e., “paucibacillary disease”), inadequate sputum specimens, or errors during specimen processing. Molecular testing (e.g., Xpert MTB/RIF) and/or more direct sampling by means of BAL and biopsy can help to make a diagnosis. In individuals in which the diagnosis is uncertain, improvement of imaging findings and symptoms after 2 months of RIPE is suggestive of drug-sensitive TB. The optimal treatment regimen for culture-negative TB has not yet been determined and remains highly controversial. Some studies have suggested that 4 months of therapy (i.e., 2 months of INH and RIF in the continuation phase, versus 4 months) may be adequate, assuming the patient is an adult who does not have HIV (53). Therefore, U.S. guidelines recommend a 4-month regimen in these individuals if there has been a clinical or radiographic response at 2 months, with a similar decision about the ability to shorten therapy at 4 months (40). However, if there are concerns about the quality of the diagnostic work-up then the fuller duration of 6 months is advised.
Conclusions
TB still places an enormous burden on health systems in resource-poor and resource-rich countries. Drug-sensitive TB is an eminently curable disease, if adequate resources are available to diagnose, evaluate and treat patients. In the preceding review, we have summarized the current care of such patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Houben RM, Dodd PJ. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med 2016;13. [Crossref] [PubMed]
- WHO. WHO Global Tuberculosis Report 2017. 2017. ISBN 978 92 4 156539 4.
- Lewinsohn DM, Leonard MK, Lobue PA, et al. Official American thoracic society/Infectious diseases society of America/Centers for disease control and prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis 2017;64:111-5. [Crossref] [PubMed]
- Choyke PL, Sostman HD, Curtis AM, et al. Adult-onset pulmonary tuberculosis. Radiology 1983;148:357-62. [Crossref] [PubMed]
- Mazurek GH, Jereb J, Vernon A, et al. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep 2010;59:1-25. [PubMed]
- Afrasiabian S, Mohsenpour B, Bagheri KH, et al. Diagnostic value of serum adenosine deaminase level in pulmonary tuberculosis. J Res Med Sci 2013;18:252-4. [PubMed]
- Khalil KF, Ambreen A, Butt T. Comparison of sensitivity of QuantiFERON-TB gold test and tuberculin skin test in active pulmonary tuberculosis. J Coll Physicians Surg Pak 2013;23:633-6. [PubMed]
- Na M. Comparison of Induced Sputum and Bronchoscopy in Diagnosis of Active Pulmonary Tuberculosis. Korean J Med 1998;55:75-82.
- Merrick ST, Sepkowitz KA, Walsh J, et al. Comparison of induced versus expectorated sputum for diagnosis of pulmonary tuberculosis by acid-fast smear. Am J Infect Control 1997;25:463-6. [Crossref] [PubMed]
- Atiq-ur-Rehman M, Naseem A, Hussain T. Comparison of diagnostic yield of AFB with sputum induction to spontaneous sputum examination in suspected pulmonary tuberculosis. J Coll Physicians Surg Pak 2009;19:506-9. [PubMed]
- Gupta BK, Bharat V, Bandyopadhyay D. Sensitivity, specificity, negative and positive predictive values of adenosine deaminase in patients of tubercular and non-tubercular serosal effusion in India. J Clin Med Res 2010;2:121-6. [PubMed]
- Fontanilla JM, Barnes A, von Reyn CF. Current diagnosis and management of peripheral tuberculous lymphadenitis. Clin Infect Dis 2011;53:555-62. [Crossref] [PubMed]
- Huhti E, Brander E, Paloheimo S, et al. Tuberculosis of the cervical lymph nodes: a clinical, pathological and bacteriological study. Tubercle 1975;56:27-36. [Crossref] [PubMed]
- Baghaei P, Tabarsi P, Farnia P, et al. Utility of gastric lavage for diagnosis of tuberculosis in patients unable to expectorate sputum. J Glob Infect Dis. 2011;3:339-43. [Crossref] [PubMed]
- Jones BE, Young SM, Antoniskis D, et al. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis 1993;148:1292-7. [Crossref] [PubMed]
- Pai M, Nicol MP, Boehme CC. Tuberculosis Diagnostics: State of the Art and Future Directions. Microbiol Spectr 2016;4:363-78. [PubMed]
- Malekmohammad M, Marjani M, Tabarsi P, et al. Diagnostic yield of post-bronchoscopy sputum smear in pulmonary tuberculosis. Scand J Infect Dis 2012;44:369-73. [Crossref] [PubMed]
- George PM, Mehta M, Dhariwal J, et al. Post-bronchoscopy sputum: Improving the diagnostic yield in smear negative pulmonary TB. Respir Med 2011;105:1726-31. [Crossref] [PubMed]
- Taylor Z, Marks SM, Rios Burrows NM, et al. Causes and costs of hospitalization of tuberculosis patients in the United States. Int J Tuberc Lung Dis 2000;4:931-9. [PubMed]
- Langer AJ, Iqbal SA, Pratt R, et al. Reported Tuberculosis in the United States, 2015. 2016:183. Available online: https://www.cdc.gov/tb/statistics/reports/2015/pdfs/2015_surveillance_report_fullreport.pdf
- Jones BE, Ryu R, Yang Z, et al. Chest radiographic findings in patients with tuberculosis with recent or remote infection. Am J Respir Crit Care Med 1997;156:1270-3. [Crossref] [PubMed]
- American Thoracic Society. Diagnostic standards and classification of tuberculosis. Am Rev Respir Dis 1990;142:725-35. [Crossref] [PubMed]
- Miller WT, Miller WT Jr. Tuberculosis in the normal host: radiological findings. Semin Roentgenol 1993;28:109-18. [Crossref] [PubMed]
- Burrill J, Williams CJ, Bain G, et al. Tuberculosis: A Radiologic Review. RadioGraphics 2007;27:1255-73. [Crossref] [PubMed]
- Singh A, Kumar VF, Anila F, et al. An unusual case of paradoxical enlargement of lymph nodes during treatment of tuberculous lymphadenitis in immunocompetent patient and literature review. Am J Case Rep 2013;14:201-4. [Crossref] [PubMed]
- Leung AN, Müller NL, Pineda PR, et al. Primary tuberculosis in childhood: radiographic manifestations. Radiology 1992;182:87-91. [Crossref] [PubMed]
- Muller NL, Franquet T, Lee KS, et al. editors. Pulmonary Tuberculosis. In: Imaging of Pulmonary Infections. Lipincott Williams & Wilkins, 2006.
- Daley C, Gotway M, Jasmer R. Radiographic Manifestations of Tuberculosis. 2nd edition. San Francisco: Curry International Tuberculosis Center, 2011.
- Alfred Weber BL, Bird KT, Janower ML. Primary tuberculosis in childhood with particular emphasis on changes affecting the tracheobronchial tree. Am J Roentgenol 1968;103:123-32. [Crossref]
- Woodring JH, Vandiviere HM, Fried AM, et al. Update: The radiographic features of pulmonary tuberculosis. AJR Am J Roentgenol 1986;146:497-506. [Crossref] [PubMed]
- Goodwin RA, Des Prez RM. Apical localization of pulmonary tuberculosis, chronic pulmonary histoplasmosis, and progressive massive fibrosis of the lung. Chest 1983;83:801-5. [Crossref] [PubMed]
- Sochocky S. Tuberculoma of the lung. Am Rev Tuberc 1958;78:403-10. [PubMed]
- Epstein DM, Kline LR, Albelda SM, et al. Tuberculous Pleural Effusions. Chest 1987;91:106-9. [Crossref] [PubMed]
- Perlman DC, El-Sadr WM, Nelson ET, et al. Variation of Chest Radiographic Patterns in Pulmonary Tuberculosis by Degree of Human Immunodeficiency Virus–Related Immunosuppression. Clin Infect Dis 1997;25:242-6. [Crossref] [PubMed]
- Allen E. Tuberculosis and other mycobacteria of the lung. In: Thurlbeck WM, Churlbeeck AM. editors. Pathology of the Lung. Second. Thieme Medical, 1995:229-65.
- Hatipoğlu ON, Osma E, Manisali M, et al. High resolution computed tomographic findings in pulmonary tuberculosis. Thorax 1996;51:397-402. [Crossref] [PubMed]
- Menzies D. Effect of treatment on contagiousness of patients with active pulmonary tuberculosis. Infect Control Hosp Epidemiol 1997;18:582-6. [Crossref] [PubMed]
- Fitzwater SP, Caviedes L, Gilman RH, et al. Prolonged Infectiousness of Tuberculosis Patients in a Directly Observed Therapy Short‐Course Program with Standardized Therapy. Clin Infect Dis 2010;51:371-8. [Crossref] [PubMed]
- Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946-1986, with relevant subsequent publications. Int J Tuberc Lung Dis 1999;3:S231-79. [PubMed]
- Nahid P, Dorman SE, Alipanah N, et al. Executive Summary: Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. Clin Infect Dis 2016;63:853-67. [Crossref] [PubMed]
- World Health Organization. Update. 2017;373:2017. [Crossref]
- Jo KW, Yoo JW, Hong Y, et al. Risk factors for 1-year relapse of pulmonary tuberculosis treated with a 6-month daily regimen. Respir Med 2014;108:654-9. [Crossref] [PubMed]
- Friedrich SO, Rachow A, Saathoff E, et al. Assessment of the sensitivity and specificity of Xpert MTB/RIF assay as an early sputum biomarker of response to tuberculosis treatment. Lancet Respir Med 2013;1:462-70. [Crossref] [PubMed]
- Gillespie SH, Crook AM, McHugh TD, et al. Four-Month Moxifloxacin-Based Regimens for Drug-Sensitive Tuberculosis. N Engl J Med 2014;371:1577-87. [Crossref] [PubMed]
- Merle CS, Fielding K, Sow OB, et al. A Four-Month Gatifloxacin-Containing Regimen for Treating Tuberculosis. N Engl J Med 2014;371:1588-98. [Crossref] [PubMed]
- DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2016. Accessed March 12, 2018. Available online: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf
- Eurosurveillance editorial team. WHO revised definitions and reporting framework for tuberculosis. Euro Surveill 2013;18:20455. [PubMed]
- Alsultan A, Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis: An update. Drugs 2014;74:839-54. Erratum in: Drugs 2014;74:2061. Dosage error in article text. [Crossref] [PubMed]
- Boyles TH, Hughes J, Cox V, et al. False-positive Xpert® MTB/RIF assays and previous treatment. Int J Tuberc Lung Dis 2015;19:495-6. [Crossref] [PubMed]
- Saukkonen JJ, Cohn DL, Jasmer RM, et al. An official ATS statement: Hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med 2006;174:935-52. [Crossref] [PubMed]
- Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents. Accessed March 12, 2018. Available online: https://aidsinfo.nih.gov/guidelines
- Ahmad Khan F, Minion J, Al-Motairi A, et al. An updated systematic review and meta-analysis on the treatment of active tuberculosis in patients with hiv infection. Clin Infect Dis 2012;55:1154-63. [Crossref] [PubMed]
- Teo SK, Tan KK, Khoo TK. Four-month chemotherapy in the treatment of smear-negative pulmonary tuberculosis: Results at 30 to 60 months. Ann Acad Med Singapore 2002;31:175-81. [PubMed]


