Deep sternal infections after in situ bilateral internal thoracic artery grafting for left ventricular myocardial revascularization: predictors and influence on 20-year outcomes
Introduction
The left internal thoracic artery (LITA) is considered as the gold-standard conduit for the revascularization of the left anterior descending artery (LAD) with higher long-term patency rates (1), enhanced survival and lower incidence of cardiac events (2). Many studies have indicated that bilateral ITA grafting (BITA) further improves survival and reduces the need for repeat revascularization (3-9). Nevertheless, many surgeons avoid bilateral ITA grafting because of the potential for an increased risk of deep sternal wound infection (DSWI). Skeletonization of the ITA has emerged as a suitable solution to sternal wound complications following BITA, allowing the preservation of the collateral blood supply to the sternum and thus reducing the incidence of parietal damage (10-16).
Risk factors for DSWI have been amply explored in the past to identify patients carrying the greatest intrinsic risk of DSWI (17-20). The association of DSWI with bilateral ITA grafting has been controversial. A number of studies have demonstrated a significant risk (21-23) whilst others have shown an increased although nonsignificant risk (24) and one study showed no risk at all (25). In addition, the true effect of BITA skeletonization on sternal perfusion and clinical outcomes remains under debate (26). Furthermore, long-term survival and the mortality profile of patients with DSWI after hospital admission have been poorly evaluated (27-29) and little is known regarding the influence of DSWI on other long-term outcomes.
Therefore, we have studied the incidence and potential factors influencing DSWI in a cohort of patients undergoing skeletonized BITA CABG using the same technique over time to test the hypothesis that this technique reduces the incidence of DSWI also in high risk patients. Finally, we explored the influence of DSWI on long-term survival, major adverse cardiac events (MACE) and repeat coronary revascularization (RCR).
Methods
Ethical Committee approval was waived due to the retrospective analysis of the study according to National Laws regulating observational retrospective studies (Italian law nr.11960, released on 13/07/2004). However, written informed consent was obtained from all the patients subjected to the surgical procedure and the treatment of data for scientific purposes which complied with Europe’s General Data Protection Regulation.
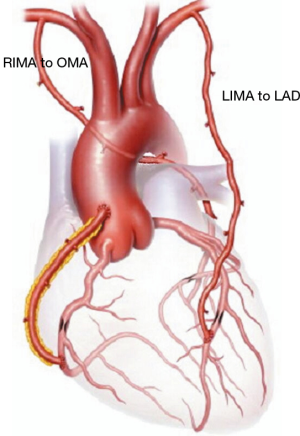
Between January 1997 and May 2017, patients undergoing coronary artery bypass grafting (CABG) with in situ skeletonized BITA to the left coronary circulation were enrolled for this study (Figure 1). Inclusion criteria were the presence of at least two angiographically branches of the left coronary circulation graftable with bilateral skeletonized ITAs. Myocardial viability under target stenotic coronary artery was detected with echocardiography, stress testing or nuclear magnetic resonance, if necessary. Patients receiving RITA on LAD, RITA on a right coronary branch, those with free or Y grafts, or those receiving a venous graft were excluded.
Clinical and instrumental data were prospectively recorded in a dedicated database. Follow-up data were collected by telephone interviews with the patients themselves or family members as well as from subsequent hospital records, the patient’s physician or outpatient clinics.
Definitions and outcomes
Primary outcome was DSWI, defined as an infection involving the muscle, bone and/or mediastinum that occurred within 30 days of surgery, required operative intervention (incision and drainage or re-exploration), had positive cultures if obtained and the patient was not previously on antibiotics at the time of sampling, and received antibiotic treatment beyond routine perioperative prophylaxis (30).
Secondary outcomes were long-term survival, MACE and RCR. MACE included cardiac death, nonfatal myocardial infarction (MI) or angina, target vessel revascularization, heart failure and stroke. RCR included repeat CABG or percutaneous coronary intervention (PCI).
Postoperative MI was defined as an increase in biomarker values above five times the 99th percentile of the normal reference range during the initial 72 hours following CABG, associated with the appearance of new pathological Q-waves or new left bundle branch block (LBBB), or angiographically documented new graft or native coronary artery occlusion, or imaging evidence of new loss of viable myocardium (31).
Surgical technique
The target for skeletonized in situ LITA was the LAD and, eventually, its branches (with sequential or composite graft). The target of the remaining left coronary circulation for skeletonized in situ RITA (Figure 1) grafting was selected as follows: in patients with two graftable branches, the branch with the larger perfusion area was selected as a primary target; if both branches had identical perfusion areas, the distal branch was selected as target for RITA grafting and the other vessel was re-vascularized with composite, sequential ITA grafts or other available conduits if indicated. In patients with heavily calcified aorta OPCAB-BITA represented first choice to reduce the risk of embolism of calcified plaques. Care was taken throughout the surgery to keep the pleurae closed, to spare the communicating bifurcation of the ITAs to the chest wall and to preserve the pericardiacophrenic artery branches. The ITAs free flow was evaluated intraoperatively and, in all cases, was >50 mL/min and therefore all ITAs were employed. In case a radial graft was planned, the artery was harvested from the non-dominant arm after a preoperative assessment of the palmar arch with the Allen test. Digital plethysmography was carried out in a number of doubtful cases.
Statistical analysis
To address missing values for variable (Table S1) we used fully conditional specification multiple imputation method (1,000 replications) (32).
The normality of distribution was assessed using the Kolmogorov-Smirnov test. Continuous data were summarized as mean and standard deviation or median and twenty-fifth to seventy-fifth percentiles in case of skewed distributions. Categorical variables were reported as counts and percentages.
Comparisons were carried out using chi-square, Fisher’s and Mann-Whitney tests where appropriate. Forty-two parameters (supplementary) were considered in the initial model for their predictive value. To enhance the accuracy of the model, the number of variables was reduced using variable clustering (33). All variables with P<0.2 at univariate analysis were introduced into multivariable logistic regression with the forward stepwise method to identify predictors of DSWI. The model was adjusted by preoperative, intraoperative, and postoperative factors. We also tested for interaction terms between potential factors for sternal complications (obesity, female sex, diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), age ≥80 years, peripheral vascular disease (PVD) (34), and a sub analysis to calculate ORs of combining factors.
The Kaplan-Meier method and log-rank test were used for survival analysis. A Cox regression model was used to estimate predictors of death. The proportional hazard assumption was confirmed by use of Schoenfeld residuals. Cumulative incidence curves were used for MACE and RCR and testing differences by the Gray test.
Finally, a competing risk analysis was used to avoid overestimation of the incidence of repeated revascularization and MACE.
R, release 3.2.3 (R Foundation for Statistical Computing, Wien, Austria) software and “survival”, “cmprsk” and “forestplot” packages were utilized. Significance for hypothesis testing was set at the 0.05 two-tailed level.
Results
Patients
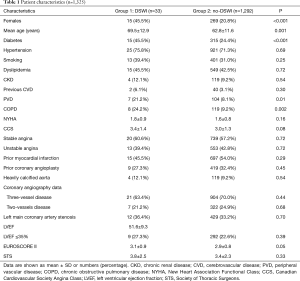
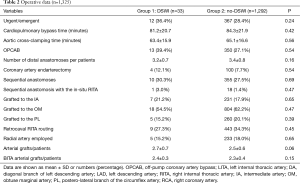
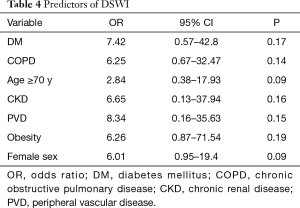
The study population consisted of 1,325 patients (Table 1). Thirty-three patients (2.5%) presented with sternal wound infection (group 1) whereas 1,292 did not (group 2). Amongst those experiencing DSWI, 12 (0.9%) required surgical treatment or surgical re-exploration while the others were treated with incision and drainage. The time to DSWI diagnosis ranged between 3 and 22 days, with a median of 11 days. After diagnosis was established, if surgical revision was necessary, debridement of necrotic tissue and topical treatment with antiseptic solution and VAC were carried out and secondary closure of the open chest wound was performed. Patients with DSWI were older (P=0.001), in a larger percentage female (P<0.001), diabetic (P<0.001), experienced PVD (P=0.01) or COPD (P=0.002). No other differences were detected amongst preoperative characteristics in the two Groups. Surgical data are given in Table 2. No differences were found between the two groups regarding surgical technique, number of anastomoses and graft geometry (all >0.5).
Full table
Full table
Early outcomes
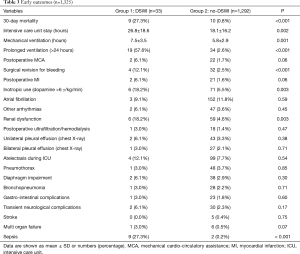
Overall 30-day mortality was 1.4% (n=19). Causes of mortality were: acute MI (n=5), aortic dissection (n=2), low cardiac output (n=2), multi-organ failure (MOF, n=4), sepsis (n=2), low-limb ischemia (n=2) and mesenteric ischemia (n=2). Early mortality was higher in group 1 (P<0.001).
Furthermore, amongst DSWI patients, mortality was significantly higher in diabetics [330/1,325 (24.9%) vs. 15/33 (45.4%), P=0.003]. Other early outcomes are shown in Table 3. Patients with DSWI had longer ICU stay and ventilation time, needed more inotropic support and experienced more revision for bleeding, renal dysfunction, MOF or sepsis.
Full table
Predictors of DSWI
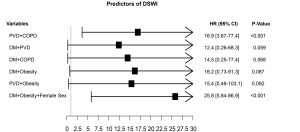
At multivariable logistic regression analysis (Table 4) found any single independent predictor of DSWI. However, we found significant interaction terms between PVD and DM (P=0.01), COPD and PVD (P<0.001), PVD and obesity (P=0.002), DM and obesity (P<0.001) and DM and COPD (P=0.008), DM and female sex (P=0.002) and female sex and obesity (P=0.001) A sub-analysis (Figure 2) showed that the association of PVD and DM increased the risk by 1.4 and 1.6 times compared to the single variables, respectively. Furthermore, when PVD occurred with COPD the risk was >2 times higher than PVD alone and 2.7 times higher than COPD. Moreover, the association with obesity increased the risk of DSWI by PVD by 1.9 times and by obesity by 2.7 times. When DM was associated with obesity the risk increased by 2.1 and 2.6 times compared to the single factors, respectively. Additionally, the association of DM and COPD increased the risk by 1.9 and 2.3 times compared to the single variables, respectively. Finally, when we tested the association between more than 2 factors, obese female patients were at a 1.6-fold higher risk when compared to the association of DM with obesity.
Full table
Long-term results
Follow up was 100% complete and ranged from 1 to 245 months. Median follow-up was 103 months (IQR, 61 to 189 months). Cumulative follow-up was 16,430 patient years.
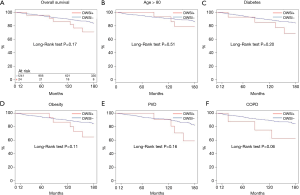
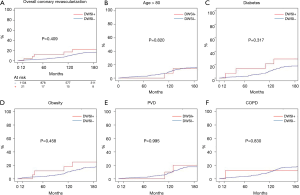
Actuarial survival at 5, 10, and 15 years were 95.3% (94.1–96.6%) vs. 95.8% (88.2–100.0%), 90.1% (88.2–92.0%) vs. 86.7% (73.7–90.0%), 83.6% (80.9–86.4%) vs. 70.9% (53.5–93.9%) (all P>0.05) in group 1 and 2, respectively (Figure 3A). In addition, there was no difference in survival between the two groups in older patients, diabetic, obese or with PVD or COPD (Figure 3B,C,D,E,F).
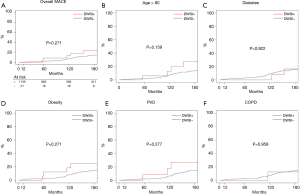
Cumulative incidence of MACE at 5, 10, and 15 years (Figure 4A), were 2.13% (1.3–2.96%) vs. 8.7% (0.3–10.53%), 7.4% (5.6–9.1%) vs. 13.0% (2.1–17.7%), 14.5% (11.9–17.1%) vs. 20.5% (3.7–34.9%) in group 1 and 2, respectively. There was no differences between the two groups (all, P>0.05) also in older, diabetic, obese patients or patients suffering from PVD or COPD (Figure 4B,C,D,E,F).
Finally, cumulative incidence of RCR at 5, 10, and 15 years were 4.96% (2.99–7.93%) vs. 10.5% (1.03–22.03%), 9.32% (7.44–11.21%) vs. 15.2% (1.42–30.82%), 16.3% (13.6–18.9%) vs. 20.0% (4.3–37.9%) in group 1 and 2, respectively. It was comparable (P>0.05) between the two groups in the whole population (Figure 5A) and also by subgroups (Figure 5B,C,D,E,F).
Predictors of late outcomes
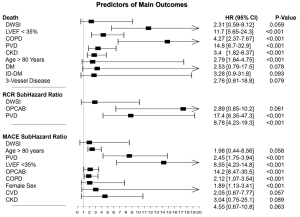
At Cox regression, LVEF <35%, COPD, PVD, chronic kidney disease (CKD) and age >80 (all, P<0.001) were predictors of death at follow-up. Furthermore, at competing risk analysis, PVD and OPCAB (both, P<0.001) predicted RCR. Finally, age >80 years, PVD, LVEF <35%, OPCAB and COPD (all, P<0.001) were independent predictors of MACE. In contrast, DSWI was not a significant predictor of death, RCR or MACE (Figure 6).
Discussion
Bilateral internal thoracic artery (BITA) has been proven to provide better event-free survival than single ITA (3-9). Nevertheless, concern about DSWI is one of the main reasons limiting the widespread use of BITA (35). Skeletonized harvesting has been proposed to minimize the risk of DSWI by preserving sternal perfusion especially in the context of BITA.
Since 1997, we have extensively employed the in-situ skeletonized BITA for left coronary circulation grafting (10,12,36,37) therefore, in this report we explored the occurrence of DSWI in our population and tried to identify factors that may predict sternal infection.
In our experience, DSWI occurred in 2.5% of patients and this is in line with data between 0.5% and 6.8 shown in the literature (38) and comparable to 1.9% incidence recently reported by the recent ART trial for the BITA patients group (39) although the skeletonized technique was employed in the ART trial in approximatively half of the patients with BITA and 46% with SITA.
The role of diabetes as a major risk factor for DSWI has been extensively studied and the prevalence of diabetes has been found to be three times higher among patients with DSWI than those without DSWI (2,39,40) although diabetic patients may actually have the most to gain from BITA grafting (41). In this study, the incidence of DSWI in diabetic patients was 4.5% (15/330) and DM did not predict DSWI, although, in case of DSWI in diabetics, the mortality raised significantly. This is in accordance with other studies (5,7,15,16,42,43). However, the skeletonized harvesting technique could be responsible for these results since it that preserves collateral blood flow to the sternum, crucial in these patients in the absence of the ITA (39).
Though, we believe that the aggressive treatment received by our DM patients referred to CABG surgery is mandatory for positive outcomes. This is in line with Lazar et al. (44) who showed that preoperative hemoglobin A1c (HbA1c) levels were not predictive of early adverse outcomes following CABG if glycemic control was achieved. Indeed some authors suggest that preoperative HbA1c measurement should be taken in all patients, since it should reflect the adequacy of glycemic control when its level is less than 7% (45). This has been confirmed by Halkos et al. (46) who found a significant association between HbA1c >7% and DSWI.
The current recommendation from the Society of Thoracic Surgeons guidelines is that oral hypoglycemics should be withheld for at least 24 h prior to surgery (45). In our practice, patients with a blood glucose concentration greater than 180 mg/dL (10.0 mmol/L) who are awaiting elective CABG receive a continuous insulin infusion to maintain their glucose concentration below 150 mg/dL (8.3 mmol/L). In these patients, we hourly measure intraoperative blood glucose concentrations, taking care to avoid perioperative hypoglycemia especially in patients with abnormal kidney function who should be promptly identified preoperatively (45). However, when DM is associated to obesity, PVD and COPD, the risk of DSWI raises by 2.1, 1.6 and 1.9 times, respectively. COPD is one of the most important risk factors for sternal dehiscence, and its prevalence is increasingly frequent in patients undergoing cardiac surgery (47). Discontinuation of smoking, clearance of secretions and prevention and relief of atelectasis, respiratory physiotherapy, including incentive spirometry, deep breathing exercises, expectoration, and bronchodilator therapy for 1 or 2 weeks before surgery and in the postoperative period are mandatory. Adopting these measures in this subset of patients led to the utilization of skeletonized- BITA CABG being safe in patients with COPD. In addition, we found obese diabetic to have a 2.1-fold greater risk of DSWI but this risk increased, in the case of obese diabetic females—1.6-fold compared to the association of DM with obesity. This confirms findings previously reported by reported by Matsa et al. (48) and colleagues and stated by Lev-Ran and colleagues (49). Although our data must be read with caution due to the higher percentage of females in the DSWI patients and the unbalance between the two groups, our results might suggest that obese diabetic women should not undergo BITA grafting. This must be confirmed by larger studies.
Moreover, to the best of our knowledge, few reports in the literature have discussed long-term results following DSWI (27). In this report, we explore the influence of DSWI on late outcomes over 20 years. A strength of our study is the long follow up that, as far as we know, is one of the longest reported in the literature.
In our experience, DSWI did not influence 15-year survival and this was confirmed also in high-risk subgroups. In addition, DSWI did not predict survival whereas LVEF <35%, COPD, PVD, CKD, no-total-arterial revascularization and age >80 years were confirmed to be independent predictors of death (3,13,14). Our findings are in accordance with Colombier et al. (29) who showed that post-sternotomy deep wound infection does not influence long-term survival in a patient-matched population. Furthermore, our data is consistent with results reported by Cayci et al. (28), showing any influence of DSWI on long-term survival. In other words, if patients survive the critical postoperative phase, their life expectancy does not differ from that of patients without DSWI. In contrast, Toumpoulis et al. (50) found that DSWI following CABG was associated with increased long-term mortality during a 10-year follow-up study. These investigators, however, included postoperative variables which are complications and hence may not be true independent predictors of DSWI. Similarly to Ridderstolpe et al. (51) and Lu et al. (52), we performed adjustment for all preoperative, intraoperative, and postoperative factors. Nonetheless, looking at our findings, the reader must keep in mind that, since we included in the analysis both deep and superficial sternal infections, this might have lowered the reported HR since superficial infections are expected to have shown lower long-term mortality rates than deep infections.
Finally, even less information is available regarding the influence of DSWI on MACE and repeat revascularization that actually represents a novel finding of our study. Deep sternal infection was not a predictive factor neither for MACE or RCR. In contrast, age >80 years, PVD, LVEF <35%, presence of OPCAB and COPD (all, P<0.001), were independent predictors of MACE whereas PVD and OPCAB (both, P<0.001) predicted RCR. We can postulate that to the higher over time patency rate of arterial graft compared to saphenous vein conduits, BITA technique is one of the main reasons of such positive outcomes and we cannot exclude that the use of skeletonized mammary artery also significantly contributed to reduction of these complications. Further research will clarify this matter.
Limitations
Our research has some limitations that need to be recognized. Firstly, the retrospective nature of the study does not enable us to draw final conclusions. Secondly, the number of DSWIs, as in most previous studies (50,53-55), was small. Thirdly, this study refers to a single-center database, and it is likely that selection of homogeneous population that represents the main strength of our study may also be a drawback since it may have introduced a selection bias. Fourthly, we did not make comparison between BITA vs. non-BITA, LITA + radial vs. LITA + SVGs etc. since this was beyond the aims of our paper. This will be, however, object of an oncoming paper. Fifthly, the target coronary vessels were determined mainly by the perfusion area of the recipient artery and not selected randomly and this may have introduced a bias. Finally, postoperative angiography was not routinely obtained therefore the patency of the grafts was unknown.
Conclusions
Skeletonized BITA, with an accurate patient selection (i.e., exclusion of obese diabetic females) and strict DM control should be considered the first choice for the majority of patients undergoing CABG due to its long-term patency and not adding a significant risk of DSWI. In addition, DSWI resulted not to be a continuing detrimental risk factor for long-term outcomes following CABG, showing that its negative effects did not extend beyond a 30-day time period. Larger randomized studies on this controversial matter are warranted.
Variables
Baseline
Sex, age, age ≥70 years, diabetes, hypertension, smoking, dyslipidemia, chronic renal disease (CKD), cerebrovascular disease (CVD), peripheral vascular disease (PVD), chronic obstructive pulmonary disease (COPD), New Heart Association Functional Class (NYHA) ≥ III, Canadian Cardiovascular Society Angina Class (CCS) ≥ III, unstable angina, prior myocardial infarction (MI), ≥ three vessel-disease, main coronary artery disease, left ventricular ejection fraction (LVEF) ≤35%, EuroScore II ≥6%, Society of Thoracic Surgeons (STS) ≥10%.
Intraoperative
Emergency, REDO, CPB time, CCT, OPCAB, number of anastomoses, coronary endarterectomy, sequential anastomosis, targeted obtuse marginal artery (OM) branch, total arterial myocardial revascularization (TAMR), composite graft.
Postoperative
ICU stay, mechanical ventilation >24 h, MI, AF, bleeding, renal dysfunction, CVVH, dialysis, stroke, MOF, surgical wound revision.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethical Committee approval was waived due to the retrospective analysis of the study according to National Laws regulating observational retrospective studies (Italian law nr.11960, released on 13/07/2004), written informed consent was obtained from all the patients.
References
- Sims FH. A comparison of coronary and internal mammary arteries and implications of the results in the etiology of arteriosclerosis. Am Heart J 1983;105:560-6. [Crossref] [PubMed]
- Taggart DP, D'Amico R, Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet (London, England) 2001;358:870-5. [Crossref] [PubMed]
- Pick AW, Orszulak TA, Anderson BJ, et al. Single versus bilateral internal mammary artery grafts: 10-year outcome analysis. Ann Thorac Surg 1997;64:599-605. [Crossref] [PubMed]
- Lytle BW, Blackstone EH, Sabik JF, et al. The effect of bilateral internal thoracic artery grafting on survival during 20 postoperative years. Ann Thorac Surg 2004;78:2005-12; discussion 2012-4.
- Dorman MJ, Kurlansky PA, Traad EA, et al. Bilateral internal mammary artery grafting enhances survival in diabetic patients: a 30-year follow-up of propensity score-matched cohorts. Circulation 2012;126:2935-42. [Crossref] [PubMed]
- Popovic B, Voillot D, Maureira P, et al. Bilateral internal mammary artery bypass grafting: long-term clinical benefits in a series of 1000 patients. Heart 2013;99:854-9. [Crossref] [PubMed]
- Takagi H, Goto SN, Watanabe T, et al. A meta-analysis of adjusted hazard ratios from 20 observational studies of bilateral versus single internal thoracic artery coronary artery bypass grafting. J Thorac Cardiovasc Surg 2014;148:1282-90. [Crossref] [PubMed]
- Yi G, Shine B, Rehman SM, et al. Effect of bilateral internal mammary artery grafts on long-term survival: a meta-analysis approach. Circulation 2014;130:539-45. [Crossref] [PubMed]
- Magruder JT, Young A, Grimm JC, et al. Bilateral internal thoracic artery grafting: Does graft configuration affect outcome? J Thorac Cardiovasc Surg 2016;152:120-7. [Crossref] [PubMed]
- Bonacchi M, Prifti E, Giunti G, et al. Respiratory dysfunction after coronary artery bypass grafting employing bilateral internal mammary arteries: the influence of intact pleura. Eur J Cardiothorac Surg 2001;19:827-33. [Crossref] [PubMed]
- Bonacchi M, Battaglia F, Prifti E, et al. Surgical revascularization of coronary bifurcations employing a single arterial graft according to the "omega-anastomosis" technique: initial experience. J Card Surg 2004;19:464-70. [Crossref] [PubMed]
- Bonacchi M, Battaglia F, Prifti E, et al. Early and late outcome of skeletonised bilateral internal mammary arteries anastomosed to the left coronary system. Heart 2005;91:195-202. [Crossref] [PubMed]
- Boodhwani M, Lam BK, Nathan HJ, et al. Skeletonized internal thoracic artery harvest reduces pain and dysesthesia and improves sternal perfusion after coronary artery bypass surgery: a randomized, double-blind, within-patient comparison. Circulation 2006;114:766-73. [Crossref] [PubMed]
- Hu X, Zhao Q. Skeletonized internal thoracic artery harvest improves prognosis in high-risk population after coronary artery bypass surgery for good quality grafts. Ann Thorac Surg 2011;92:48-58. [Crossref] [PubMed]
- Sá MP, Ferraz PE, Escobar RR, et al. Skeletonized versus pedicled internal thoracic artery and risk of sternal wound infection after coronary bypass surgery: meta-analysis and meta-regression of 4817 patients. Interact Cardiovasc Thorac Surg 2013;16:849-57. [Crossref] [PubMed]
- Suzuki T, Asai T, Nota H, et al. Similar outcome in insulin-dependent and noninsulin-dependent diabetic patients after off-pump coronary artery bypass grafting with multiple skeletonized arterial conduits. Ann Thorac Surg 2015;99:1562-7. [Crossref] [PubMed]
- Heilmann C, Stahl R, Schneider C, et al. Wound complications after median sternotomy: a single-centre study. Interact Cardiovasc Thorac Surg 2013;16:643-8. [Crossref] [PubMed]
- Milano CA, Kesler K, Archibald N, et al. Mediastinitis After Coronary Artery Bypass Graft Surgery. Risk Factors and Long-term Survival Circulation 1995;92:2245-51. [PubMed]
- Parissis H, Al-Alao B, Soo A, et al. Risk analysis and outcome of mediastinal wound and deep mediastinal wound infections with specific emphasis to omental transposition. J Cardiothorac Surg 2011;6:111. [Crossref] [PubMed]
- Itagaki S, Cavallaro P, Adams DH, et al. Bilateral internal mammary artery grafts, mortality and morbidity: an analysis of 1 526 360 coronary bypass operations. Heart 2013;99:849-53. [Crossref] [PubMed]
- Kouchoukos NT, Wareing TH, Murphy SF, et al. Risks of bilateral internal mammary artery bypass grafting. Ann Thorac Surg 1990;49:210-7. [Crossref] [PubMed]
- Grossi EA, Esposito R, Harris LJ, et al. Sternal wound infections and use of internal mammary artery grafts. J Thorac Cardiovasc Surg 1991;102:342-6; discussion 346-7. [PubMed]
- Parisian Mediastinitis Study Group. Risk factors for deep sternal wound infection after sternotomy: A prospective, multicenter study. J Thorac Cardiovasc Surg 1996;111:1200-7. [Crossref] [PubMed]
- Loop FD, Lytle BW, Cosgrove DM, et al. Sternal wound complications after isolated coronary artery bypass grafting: Early and late mortality, morbidity, and cost of care. Ann Thorac Surg 1990;49:179-86. [Crossref] [PubMed]
- Galbut DL, Traad EA, Dorman MJ, et al. Seventeen-year experience with bilateral internal mammary artery grafts. Ann Thorac Surg 1990;49:195-201. [Crossref] [PubMed]
- Cheng K, Rehman SM, Taggart DP. A Review of Differing Techniques of Mammary Artery Harvesting on Sternal Perfusion: Time for a Randomized Study? Ann Thorac Surg 2015;100:1942-53. [Crossref] [PubMed]
- Braxton JH, Marrin CAS, McGrath PD, et al. Mediastinitis and long-term survival after coronary artery bypass graft surgery. Ann Thorac Surg 2000;70:2004-7. [Crossref] [PubMed]
- Cayci C, Russo M, Cheema FH, et al. Risk analysis of deep sternal wound infections and their impact on long-term survival: a propensity analysis. Ann Plast Surg 2008;61:294-301. [Crossref] [PubMed]
- Colombier S, Kessler U, Ferrari E, et al. Influence of deep sternal wound infection on long-term survival after cardiac surgery. Med Sci Monit 2013;19:668-73. [Crossref] [PubMed]
- Shahian DM, Jacobs JP, Edwards FH, et al. The Society of Thoracic Surgeons National Database. Heart 2013;99:1494. [Crossref] [PubMed]
- Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation 2007;116:2634-53. [Crossref] [PubMed]
- Liu Y, De A. Multiple Imputation by Fully Conditional Specification for Dealing with Missing Data in a Large Epidemiologic Study. Int J Stat Med Res 2015;4:287-95. [Crossref] [PubMed]
- Romesburg HC. Cluster analysis for researchers. Morrisville, NC: Lulu Press, 2004.
- Borger MA, Rao V, Weisel RD, et al. Deep sternal wound infection: risk factors and outcomes. Ann Thorac Surg 1998;65:1050-6. [Crossref] [PubMed]
- Tabata M, Grab JD, Khalpey Z, et al. Prevalence and variability of internal mammary artery graft use in contemporary multivessel coronary artery bypass graft surgery: analysis of the Society of Thoracic Surgeons National Cardiac Database. Circulation 2009;120:935-40. [Crossref] [PubMed]
- Bonacchi M, Prifti E, Giunti G, et al. Right Y-graft, a new surgical technique using mammary arteries for total myocardial revascularization. Ann Thorac Surg 2000;70:820-3. [Crossref] [PubMed]
- Prifti E, Bonacchi M, Frati G, et al. Lambda graft with the radial artery or free left internal mammary artery anastomosed to the right internal mammary artery: flow dynamics. Ann Thorac Surg 2001;72:1275-81. [Crossref] [PubMed]
- Cotogni P, Barbero C, Rinaldi M. Deep sternal wound infection after cardiac surgery: Evidences and controversies. World J Crit Care Med 2015;4:265-73. [Crossref] [PubMed]
- Taggart DP, Altman DG, Gray AM, et al. Randomized Trial of Bilateral versus Single Internal-Thoracic-Artery Grafts. NEJM 2016;375:2540-9. [Crossref] [PubMed]
- Benedetto U, Altman DG, Gerry S, et al. Pedicled and skeletonized single and bilateral internal thoracic artery grafts and the incidence of sternal wound complications: Insights from the Arterial Revascularization Trial. J Thorac Cardiovasc Surg 2016;152:270-6. [Crossref] [PubMed]
- Stevens LM, Carrier M, Perrault LP, et al. Influence of diabetes and bilateral internal thoracic artery grafts on long-term outcome for multivessel coronary artery bypass grafting. Eur J Cardiothorac Surg 2005;27:281-8. [Crossref] [PubMed]
- Toumpoulis IK, Theakos N, Dunning J. Does bilateral internal thoracic artery harvest increase the risk of mediastinitis? Interact Cardiovasc Thorac Surg 2007;6:787-91. [Crossref] [PubMed]
- Medalion B, Mohr R, Frid O, et al. Should bilateral internal thoracic artery grafting be used in elderly patients undergoing coronary artery bypass grafting? Circulation 2013;127:2186-93. [Crossref] [PubMed]
- Lazar HL, Chipkin SR, Fitzgerald CA, et al. Tight Glycemic Control in Diabetic Coronary Artery Bypass Graft Patients Improves Perioperative Outcomes and Decreases Recurrent Ischemic Events. Circulation 2004;109:1497-502. [Crossref] [PubMed]
- Reddy P, Duggar B, Butterworth J. Blood glucose management in the patient undergoing cardiac surgery: A review. World J Cardiol 2014;6:1209-17. [Crossref] [PubMed]
- Halkos ME, Puskas JD, Lattouf OM, et al. Elevated preoperative hemoglobin A1c level is predictive of adverse events after coronary artery bypass surgery. J Thorac Cardiovasc Surg 2008;136:631-40. [Crossref] [PubMed]
- Okutan H, Tenekeci C, Kutsal A. The Reinforced Sternal Closure System® Is Reliable to Use in Elderly Patients. J Card Surg 2005;20:271-3. [Crossref] [PubMed]
- Matsa M, Paz Y, Gurevitch J, et al. Bilateral skeletonized internal thoracic artery grafts in patients with diabetes mellitus. J Thorac Cardiovasc Surg 2001;121:668-74. [Crossref] [PubMed]
- Lev-Ran O, Mohr R, Amir K, et al. Bilateral internal thoracic artery grafting in Insulin-Treated diabetics: should it be avoided? Ann Thorac Surg 2003;75:1872-7. [Crossref] [PubMed]
- Toumpoulis IK, Anagnostopoulos CE, DeRose JJ, et al. The Impact of Deep Sternal Wound Infection on Long-term Survival After Coronary Artery Bypass Grafting. Chest 2005;127:464-71. [Crossref] [PubMed]
- Ridderstolpe L, Gill H, Granfeldt H, et al. Superficial and deep sternal wound complications: incidence, risk factors and mortality. Eur J Cardiothorac Surg 2001;20:1168-75. [Crossref] [PubMed]
- Lu JCY, Grayson AD, Jha P, et al. Risk factors for sternal wound infection and mid-term survival following coronary artery bypass surgery. Eur J Cardiothorac Surg 2003;23:943-9. [Crossref] [PubMed]
- Noyez L, van Druten JAM, Mulder J, et al. Sternal wound complications after primary isolated myocardial revascularization: the importance of the post-operative variables. Eur J Cardiothorac Surg 2001;19:471-6. [Crossref] [PubMed]
- Slaughter MS, Olson MM, Lee JT, et al. A fifteen-year wound surveillance study after coronary artery bypass. Ann Thorac Surg 1993;56:1063-8. [Crossref] [PubMed]
- Zacharias A, Habib RH. Factors Predisposing to Median Sternotomy Complications: Deep vs Superficial infection. Chest 1996;110:1173-8. [Crossref] [PubMed]


