Emerging technology: electrical stimulation in obstructive sleep apnoea
Introduction
Obstructive sleep apnoea (OSA) is the most common form of sleep-disordered breathing (1), defined as a clinical condition in which there is intermittent and repeated upper airway (UAW) collapse during sleep which results in irregular breathing at night and, typically, excessive sleepiness during the day.
Although the first empirical description is attributed to Charles Dickens in 1836, OSA and its associated polysomnographic findings was defined in 1965 by Jung and Kuhlo (2) in Germany and Gastaut (3) in France.
The prevalence of OSA is a worldwide burden on public health, it affected 4% of middle aged men and 2% of middle aged women in the United States in the early 1990’s (4), but with an increase in obesity rates prevalence is now 10% of the 30-49 year-old men and 3% of the 30-49 year-old women (5).
OSA is associated with significant co-morbidities, which include hypertension (6), ischaemic heart disease (7), stroke (8), congestive heart failure (9), obesity and metabolic syndrome (10), and diabetes (11). It has been recognised as a significant cardiovascular risk. Treatment of OSA is not only provided to control day- and night-time symptoms but also to reduce the overall long-term cardiovascular risks.
According to current guidelines (12), treatment with continuous positive airway pressure (CPAP) is indicated in patients with moderate-severe OSA, and a mandibular advancement device (MAD) should be considered in patients with mild symptomatic OSA. However, although CPAP provides the best effective treatment, it is not well tolerated by all patients over longer periods and the adherence rate is limited and influenced by symptoms and disease severity; approximately 2/3 of patients who should be on CPAP continue with the treatment at five years (13). Thus, alternatives to CPAP are needed for patients who fail this treatment and who continue to experience symptoms with associated cardiovascular risks.
Since 2011, a technology has emerged as a rapidly developing and promising treatment alternative in OSA. Electrical stimulation (ES) has been used to activate the dilator muscles of the UAW and this might enable patients to maintain a patent UAW while asleep. The different methods that are currently used in a research or clinical scenario, ‘pros’ and ‘cons’ and the clinical relevance of these novel treatments will be discussed.
Pathophysiology of obstructive sleep apnoea
The anatomy of the UAW is complex as it includes different groups of muscles that are involved in different physiological functions. The UAW can be divided into three compartments: (I) the naso-pharynx (epi-pharynx) whose function is mainly respiratory; (II) the oro-pharynx which has respiratory, swallow and reflex functions; and (III) the laryngo-pharynx (hypo-pharynx) whose functions include speech, swallow and respiratory function.
Neural drive to the UAW dilator muscles falls with sleep onset. The reduced activity of the UAW dilators results in a reduced neuromuscular tone with a narrowed pharyngeal lumen, which can lead to a complete collapse of the airway via the Bernoulli effect (14). This, in turn leads to snoring and, with complete obstruction, to sleep apnoea.
Remmers et al. described repetitive UAW occlusion in sleep (15) and examined the relationship between the genioglossal electromyogram (EMG) and the pharyngeal pressure demonstrating that airway occlusion occurred when the negative pharyngeal pressure exceeded the genioglossus force, a so-called ‘balance of forces’ (Figure 1).
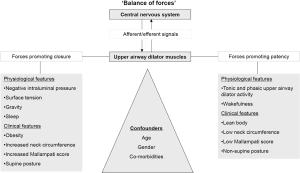
More recently, other theories have tried to explain sleep apnoea with an impaired neuromuscular response during UAW occlusion and therefore a failure to compensate and restore the airway patency. Mezzanotte et al. (16) have demonstrated that with sleep onset pharyngeal collapse is associated with a loss of genioglossus muscle (GG) tone.
Another theory hypothesises about structural defects that increase UAW collapsibility and predispose to airflow obstruction during sleep. This hypothesis has been studied by several authors (17) who showed the importance of rostral movements of the trachea during lung deflation, that are associated with reductions in longitudinal tension within the airway that can, in part, explain the pathogenesis of OSA.
Following on from the first descriptions of OSA multiple studies have attempted to describe the activity of the most important pharyngeal dilator muscles, in particular the activity of the genioglossus (18). Anatomical features and mechanical narrowing of the UAW play an important role in OSA but functional factors are similarly fundamental contributors to UAW patency (Figure 1).
Oliven’s group could show that enhanced UAW muscle activity during ES decreases UAW resistance in an isolated UAW model confirming the role of functional factors (19,20). Van de Graaff and colleagues could show that ES of the UAW dilator muscles could shift the hyoid bone anteriorly confirming the contributory role of the UAW anatomy (21).
Non-invasive methods
Submental transcutaneous ES
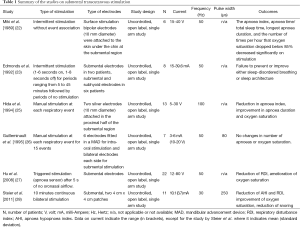
Treating the functional loss of the neuromuscular tone with sleep onset and the contributing anatomical factors at the same time in order to keep the UAW patent during sleep is hypothetical way to treat the cause of OSA. First studies on transcutaneous ES were performed by Miki et al. with promising results (22). They studied six patients with full polysomnography on and off electrical transcutaneous stimulation using 10 mm bipolar electrodes to the skin of the patients in the submental region and stimulated the patients using an apnoea demand-type stimulator. When apnoeas lasted for longer than 5 s the device delivered ES with a frequency of 50 Hz and a voltage of 15-40 V until breathing resumed or after 10 s at the longest. This kind of treatment resulted in a significant reduction of the apnoea index and the apnoea time per total sleep time.
However, shortly after this study another group could not confirm these results. Indeed, Edmonds et al. reported that transcutaneous and submental ES failed to enlarge the UAW, as observed by computer tomography during wakefulness, and they were unable to reverse UAW obstruction using this method in the asleep patient without causing arousals (23). Similar results were reported by Decker et al. (24) stating that submental transcutaneous stimulation led invariably to arousal from sleep.
A few years later, Hida et al. demonstrated the effectiveness of submental transcutaneous stimulation in patients with severe OSA (25). They stimulated 13 patients during an overnight study with ES starting when an apnoea lasted for five seconds; stimulation was stopped after ventilation resumed or after a maximum of 20 seconds. In their study, the apnoea-hypopnoea-index dropped from a mean of 53.8/h to 6.6/h.
A year later, Guilleminault et al. (26) tested submental and intra-oral sublingual ES in seven patients with severe OSA without achieving any benefit. They delivered an ad-hoc stimulation during 15 obstructive respiratory events testing bilateral and unilateral stimulation; there was no improvement compared with the baseline measurements.
More recently, two other groups have published encouraging results using ES in the submental area. Hu et al. (27) used a biphasic electrical nerve stimulator which consisted of an electrical pulse generator, an apnoea sensor and percutaneous electrodes. They enrolled 22 patients with severe OSA and tested the device during a split night study. Their device was apnoea-triggered and delivered ES if no nasal flow was detected for at least 5 s. The mean respiratory disturbance index (RDI) decreased from 30.9/h to 12.4/h while stimulation was delivered without significant changes in the micro-arousal index.
Steier et al. observed a similar efficacy when testing transcutaneous and continuous ES of the UAW muscles delivering a low current in 11 obese patients with moderate-severe OSA delivered by two large patchy electrodes (4 cm × 4 cm). After proving the effect of the stimulation on the contraction of the tongue in healthy subjects, they showed that the ES reduced the RDI from and average of 28.1/h to 10.2/h without awakening the patient, the apnoea hypopnoea index (AHI) was similarly reduced. The electrical current led to an improved oxygenation and reduced snoring (Table 1) (28).
Full table
None of the studies published on non-invasive ES in OSA has been a randomised controlled trial. Moreover, complex and variable stimulation settings make it difficult to standardise this treatment and to optimise treatment efficacy (Table 1). Given the diversity of patients and the presence of various confounders which can affect the effectiveness of the stimulation (skin impedance, neck circumference and body mass index (BMI), shape of the UAW) patient selection becomes crucial for future trials using this method to provide a tailored approach and increase the efficacy of this method. A non-invasive trial of this method to select and stratify OSA patients into responders and non-responders, for example during a drug-induced sleep endoscopy (DISE), could prove to be helpful prior to commencing discussions about an implanted stimulator.
Invasive methods
Fine wire stimulation
Initial experiments in cats, dogs and rats using direct stimulation of the GG evoked a muscle contraction and revealed improvements in the pharyngeal patency; these results prompted investigators to test this technique in humans (29-31). Bishara et al. tested ES using Teflon-coated stainless steel electrodes inserted into the genioglossus, geniohyoid, sternohyoid, and sternothyroid muscles bilaterally in 16 dogs during UAW obstruction (32). They could show that stimulation of the genioglossus and geniohyoid reduced the UAW resistance prior to and during partial occlusion which was caused by a small rubber balloon connected to a thin tube that was implanted percutaneously under the pharyngeal submucosa. More interestingly, they found that the GG was the most effective dilator muscle reducing UAW resistance and avoiding airway occlusion. In addition, changes in head, lower jaw and tongue position were shown to substantially affect the airway resistance. This observation adds complexity to the issue of UAW patency in sleep. In order to maintain a patent airway using ES the intensity would require an adjustment according to the airway resistance, influenced for example by the head posture, but at the same time changes in the electrical current should not cause arousal from sleep.
Studies on selective intra-muscular stimulation of the hyoglossus, styloglossus and GGs (33) found that there are differences in the response and the contraction of the muscles which depend on the site of the stimulation. Stimulation near the proximal hypoglossal nerve can cause a closure of the upper airway while distal hypoglossal stimulation induces a contra-lateral tongue deviation and variable degrees of airway opening during sleep (33). This might be an explanation for the variable response of the muscles during ES; it confirms that different stimulation sites will recruit different groups of muscle fibres. Proximal hypoglossal stimulation will reach the lingual muscles, pulling the base of the tongue posteriorly and distal stimulation will reach the genioglossus and moves the tongue anteriorly to sustain UAW patency.
Hypoglossal nerve stimulation (HNS)
Following publication of the results of initial experimental studies on UAW dilator muscle stimulation in OSA patients (23,34,35) several research groups started to test the possibility of stimulating the muscle via the hypoglossal nerve. A problem had arisen from the previous observations, stimulating a single protrusor muscle like the GG could result in the antagonistic activation of other neck and tongue muscles which could evoke an antagonistic effect on airway patency. However, stimulating the hypoglossal nerve could also lead to the stimulation of multiple tongue muscles, which could lead to a synergistic effect and a favorable patency of the UAW (35).
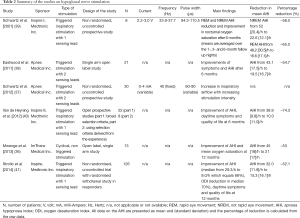
Since the beginning of the new millennium several studies have been published that demonstrate the effect of HNS in patients with OSA with consistent results in terms of effectiveness but, until recently, not in terms of patient safety (36-38). Different types of invasive stimulators were used with different specifications and stimulation settings (Table 2). However, most systems included a circumferential nerve cuff electrode, a stimulation lead and an implantable pulse generator.
Full table
In 2001, Schwartz et al. (39) published a pilot study on HNS on eight patients with OSA in whom a stimulator device (Medtronic Inc, Minnesota, MN/USA) was implanted and placed in an infra-clavicular subcutaneous pocket to deliver an inspiratory-triggered stimulation. They followed patients for a 6-month period and showed that the AHI had improved by 55-65% (Table 2).
More recently, Schwartz et al. tested a slightly different approach (37) in an observational, non-randomised and uncontrolled study using an implantable device (HGNS, Apnex Medical Inc, St Paul, MN/USA) delivering triggered stimulation to a group of 30 patients with an AHI greater than 20/h. This study revealed an improved inspiratory airflow that was dose-dependent on increasing stimulation intensity.
Eastwood et al. (38) published a single-arm open label study using the same device (HGNS, Apnex Medical Inc., St Paul, MN/USA) on 21 patients with moderate-severe OSA. At 6-month follow up the AHI had decreased by 55% with a significant improvement in daytime symptoms, the Epworth Sleepiness Scale score (ESS) was 12.1 (4.7) points at baseline and 8.1 (4.4) points at six months’ (P<0.001). However, at least one adverse event related to the implantation procedure or related to the treatment occurred in 71% and 67% of the patients, respectively.
Mwenge et al. (36) undertook a similar study to Schwartz et al. (37) but they used a different implantable system (ImThera Medical Inc, San Diego, CA/USA) which consisted of an implanted pulse generator and a multi-electrode stimulation lead. The device was implanted in 13 patients with OSA and the treatment achieved a reduction of the overall AHI from 45 [18]/h to 21 [17]/h at 12 months, a reduction of 53%, without observing any changes in the sleep architecture. An improved symptomatic response was reported with a reduction in the ESS only at 3-month follow up [ESS 10.8 (6.2) improved to 6.7 (5.4) points; P<0.05]. During the follow up period there were multiple adverse events: one patient had a defective device, two patients had transient ipsi-lateral tongue hemi-paresis, one patient underwent surgery but the device could not be implanted due to a defect, one patient had post-operative swelling of the neck, in one patient three leads broke, one patient required a repeat operation and re-implantation and his replaced lead broke at the end of the study, one patient had a Twiddler’s phenomenon (electrode displacement) and all patients experienced one or more technical adverse event.
In early 2014, Strollo et al. (41) could confirm the feasibility and effectiveness of invasive HNS in OSA. The authors implanted a stimulator device (Inspire Medical Systems, Maple Grove, MN/USA) in 126 patients with moderate-severe OSA. The patients were assessed at baseline and after 12-months using a polysomnography. Responders to the treatment (46 patients) were randomised in a 1:1 ratio to (I) a withdrawal group in which the device was turned off; and (II) a therapy maintenance group with the device turned on. The results demonstrated a reduction in the median AHI of 68% at 1 year. A symptomatic improvement in daytime sleepiness and quality of life were also reported. The withdrawal sub-study confirmed that the effect was related to ES, the mean AHI in the withdrawal group increased significantly compared to the maintenance group (AHI 25.9/h vs. 8.9/h; P<0.001). Compared to earlier studies (36) this trial (STAR; 41) revealed that the intervention was safe with a percentage of serious adverse events lower than 2%.
However, some issues need to be addressed before this treatment should be offered in the clinical setting: Firstly, 929 patients were screened for the STAR trial (41), the device was implanted in only 126 (13.6%) of the screened patients. The number of responders, defined as a reduction of at least 50% from baseline in the AHI plus an AHI after one year of less than 20 events/hour, was 46 out of 126 (36.5%). Therefore, the number of responders was less than 5% of the screened population. Secondly, there lacked randomisation and a control group in the first part, which was acknowledged by the authors. Although it is difficult to design a suitable control group, there was no consideration given to a sham group either. Thirdly, it is worth to consider the cost-effectiveness of this treatment, in particular when compared to established standard treatment (CPAP). It is likely that public healthcare will not be able to fund this treatment for a highly prevalent condition. Lastly, further studies are required to demonstrate a long-term effect of this treatment on cardiovascular risks, its impact on other co-morbidities and whether its efficacy will match the benefit of CPAP.
Discussion
Recent improvements in hardware, methodology and signal processing have led to a realistic approach of using ES of the UAW dilator muscles in patients with OSA. This method might benefit selected patients who fail standard therapy (42) due to poor long term compliance (43). Alternatives to CPAP therapy are required as the prevalence of sleep apnoea continues to rise, in line with obesity rates (44). With the current level of evidence, invasive HNS seems to be more efficacious than a non-invasive approach, but the invasive procedure, costs and complications remain a limiting factors.
Despite the progress up to date, multiple variables remain unknown. None of the trials in this field was conducted with a control group. Further, ES might reduce the severity of sleep-disordered breathing, but it remains an exclusive achievement of CPAP therapy to entirely abolish nocturnal apnoeas. A significant difference in the treatment is that CPAP effectively treats apnoeas and hypopnoeas via a ‘pneumatic splint’. In contrast, ES of the UAW maintains the neuromuscular tone of the UAW dilator muscles.
Rodenstein et al. (45) described the residual effects of ES on the UAW as a ‘disease-modifying’ concept. They observed that, when patients enrolled in the trial of HNS had to stop the treatment because of hardware failures, they remained free of symptoms for several nights. The hypothesis that HNS has a residual effect on the UAW was tested further: At one year follow up, patients who had experienced a significant improvement in the AHI with stimulation revealed similar results in the first night off treatment, the AHI and the micro-arousal index remained unchanged in the polysomnography. These findings suggest that the tonic activity of the HNS may have altered the neural pattern of the muscular tone of the UAW dilator muscles. Further studies are required to understand whether this is a lasting effect and to understand how central pathways could be altered to impact on UAW patency.
There continues to be a lack of data on long-term benefits of treatment with ES, in particular with respect to cardiovascular interactions. So far, only Strollo et al. (41) showed a mild improvement in the diastolic blood pressure from 81.5 (9.7) to 79.3 (9.5) mmHg (P=0.02) without significant changes in systolic blood pressure, heart rate and BMI.
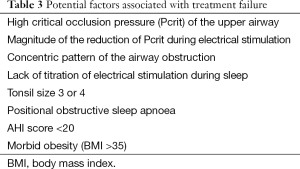
In order to employ ES in a clinical scenario patient selection remains important and methods should be developed to identify responders to this treatment prior to the intervention and to understand their characteristics; this would allow to exclude potential non-responders from an invasive intervention (Table 3). In studies on invasive and non-invasive ES patients were more likely to respond if they had moderate-severe OSA, if they were moderately obese and aged 35-50 years. There are sparse data on women, older patients and patients with mild OSA. The complexity of the musculature involved in maintaining UAW patency adds to the difficulty to accurately predict and understand the effect of ES (46).
Full table
UAW collapsibility and, more specifically, the critical occlusion pressure (Pcrit) of the UAW determine the likelihood of treatment success; a high Pcrit at baseline (24) and the magnitude of the reduction of Pcrit during ES (47) are important predictors. Similarly, the shape of the pharyngeal lumen seems to be important, a concentric pattern of the UAW obstruction was associated with a poor response to ES compared to other patterns (40).
Moreover, Vanderveken et al. (48) evaluated the possible value of DISE in the assessment of a therapeutic response to implanted UAW stimulation for patients with OSA. They found that patients with complete concentric collapse at the palatal level were less likely to benefit from HNS. DISE might also be a suitable approach to test non-invasive ES.
Further, titration trials of ES during sleep are required to determine who responds to this treatment (37) and this should include an optimisation of the stimulation settings without causing arousal (current, frequency, pulse width).
Almost all invasive devices deliver triggered stimulation during inspiration, while transcutaneous stimulation was commonly used for longer than a single breathe, because sudden changes in the current are more likely to cause skin sensation and, subsequently, arousal from sleep. However, stimulating the muscle specifically during the inspiratory phase might maximise the response (31). The only study using non-triggered invasive HNS was published by Mwenge and colleagues (36) demonstrating that continuous and non triggered stimulation was similarly effective in patients with OSA when compared to triggered stimulation.
Currently, there are various different ways to stimulate the UAW dilator muscles:
- Invasively and non-invasively;
- Triggered by physiological variables (e.g., diaphragm movement, airflow, rib cage movement), intermittent and cyclical or continuous stimulation;
- High and low intensity current;
- Nerve and direct muscle stimulation;
- Different frequencies of the current (up to 100 Hz);
- Different pulse widths and shapes;
- Unilateral and bilateral location;
- Unipolar and bipolar current.
The heterogeneity of the different study designs that were published and the variety of different stimulation settings leaves in question how best to decide which one of these techniques will be the most promising approach (Table 4), most of all because some of the results conflict with each other.

Full table
Transcutaneous ES has been used intermittently or continuously, while most invasive devices deliver the stimulation triggered by an inspiration. In the non-invasive approach, it remains crucial to avoid skin irritation and pain. Low currents need to be applied over longer periods to avoid sudden changes in the current that could awake the patient. However, in order to avoid fatigue of the muscles continued stimulation should not be applied for the entire night and an optimal on-off regime of the stimulation remains to be determined.
A compromise in the stimulation frequency needs to be found to generate a stimulation that can be maintained over several minutes with sufficient force, but without experiencing a quick fatigue (50,51). On the other hand, stimulating the muscle in a specific time-related fashion, as frequently used for the invasive approach, could maximise the response during the inspiratory phase (31). However, it has been shown that invasive (36) and non-invasive (28) continuous stimulation without a trigger could effectively reduce the severity of sleep-disordered breathing. Different stimulation frequencies, wave-forms and voltage of the stimulation current require further investigations in order to achieve the most effective response (34); a standardised stimulation frequency and an optimal wave-form or amplitude have yet to be defined.
The consideration of specific stimulation settings is fundamental in order to avoid arousal from sleep. From this perspective, invasive stimulation of the hypoglossal nerve is less likely to cause arousal from sleep than the non-invasive and transcutaneous approach, because lower currents can be used and skin irritation is largely bypassed. Intra-muscular stimulation techniques might also be beneficial because this approach allows the recruitment of selected fibres of the muscle to avoid a sensory activation on the skin receptors which could lead to an arousal.
A potential limitation to the invasive HNS is the occurrence of adverse events and side effects. Whilst the non-invasive approach has limited side effects, adverse events of the invasive method are more common and often associated with the surgical procedure. Problems have been described including anaesthesia and analgesia, surgical risks, wound infections, haematomas and nerve palsy, broken leads or incomplete nerve contact.
Invasive and non-invasive techniques share the potential long-term problems of muscle stimulation which include muscle fatigue, changes in fibre type composition and muscle hypertrophy. These effects have not been studied with these methods so far and, moreover, age (52) and body weight (53) can affect UAW collapsibility in patients treated with ES, which requires further examination.
Lastly, the hypoglossal nerve innervates various extrinsic and intrinsic muscles involved in maintaining UAW patency (54) and it is important to target the most suitable fibres innervating the muscles with a dilatory effect of the UAW and to avoid the stimulation of the muscles with antagonistic effect to the UAW patency (55).
The described progress in the development of this technique and the stimulator devices will provide us with a more accurate picture and guidance for the future; more detailed data and background information on optimal stimulation settings, titration manoeuvres and operating techniques (56) will lead to a reduction in the occurrence of adverse events and an increase in the effectiveness of the delivered stimulation.
Conclusions and perspectives
ES of the UAW dilator muscles is not a new approach to treat OSA, but recent years have seen a renaissance of this method. Several studies from different groups have triggered a steady rise in the interest in this topic. Prospective trials, although still lacking a sham-controlled and randomised approach, have shown the potential of ES; currently, there is more evidence for the efficacy of the invasive approach. However, there are encouraging results from feasibility studies published for the non-invasive approach that indicate a similar effect size to HNS. For the first time, we experience that this technology might develop into a clinical alternative to CPAP.
It is likely that in the view of the current evidence international societies will need to review and update their guidance and recommendation on the use of ES as a part of an evidence-based treatment approach in OSA (49). Despite this the cost-effectiveness and safety of this treatment remains to be further defined. Moreover, the long-term acceptance and the cardiovascular impact of UAW dilator muscle stimulation have not been sufficiently assessed. Numerous questions remain in order to identify optimal stimulation settings and to understand the best treatment approach (Table 5).

Full table
Review criteria
References for this review were retrieved from the PUBMED-MEDLINE databases. Search terms included “electrical stimulation in sleep apnoea”, “hypoglossal stimulation”, “genioglossal stimulation”, “alternative treatment for sleep apnoea” and “implantable device for sleep apnoea”. Most papers considered were full-text papers published in English language between 1978 and 2014.
Acknowledgements
Dr. Pengo’s research on electrical stimulation in OSA has been funded by the Italian Hypertension Society. Dr. Pengo also wants to acknowledge Prof. GP Rossi for his support.
Competing interests: The authors are part of the study team that undertake a trial of non-invasive electrical stimulation of the upper airway dilator muscles in obstructive sleep apnoea sponsored by Guy’s & St Thomas’ NHS Foundation Trust and King’s College London School of Medicine, the NIHR-CLRN South London, and the Italian Hypertension Society.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Steier J, Martin A, Harris J, et al. Predicted relative prevalence estimates for obstructive sleep apnoea and the associated healthcare provision across the UK. Thorax 2014;69:390-2. [PubMed]
- Jung R, Kuhlo W. Neurophysiological studies of abnormal night sleep and the Pickwickian syndrome. Prog Brain Res 1965;18:140-59. [PubMed]
- Gastaut H, Tassinari CA, Duron B. Polygraphic study of diurnal and nocturnal (hypnic and respiratory) episodal manifestations of Pickwick syndrome. Rev Neurol (Paris) 1965;112:568-79. [PubMed]
- Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230-5. [PubMed]
- Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177:1006-14. [PubMed]
- Narkiewicz K, Somers VK. Obstructive sleep apnea as a cause of neurogenic hypertension. Curr Hypertens Rep 1999;1:268-73. [PubMed]
- Martinez D, Klein C, Rahmeier L, et al. Sleep apnea is a stronger predictor for coronary heart disease than traditional risk factors. Sleep Breath 2012;16:695-701. [PubMed]
- Palomäki H, Partinen M, Juvela S, et al. Snoring as a risk factor for sleep-related brain infarction. Stroke 1989;20:1311-5. [PubMed]
- Bradley TD, Rutherford R, Grossman RF, et al. Role of daytime hypoxemia in the pathogenesis of right heart failure in the obstructive sleep apnea syndrome. Am Rev Respir Dis 1985;131:835-9. [PubMed]
- Lévy P, Bonsignore MR, Eckel J. Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J 2009;34:243-60. [PubMed]
- Punjabi NM, Sorkin JD, Katzel LI, et al. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med 2002;165:677-82. [PubMed]
- NICE technology appraisal guidance 139. CPAP for OSA costing template and report. 2008. Available online: http://www.healthcareimprovementscotland.org/idoc.ashx?docid=18137349-a5bd-42ee-992a-7d954474856e&version=-1
- McArdle N, Devereux G, Heidarnejad H, et al. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 1999;159:1108-14. [PubMed]
- Knudson RJ, Knudson DE. Effect of muscle constriction on flow-limiting collapse of isolated canine trachea. J Appl Physiol 1975;38:125-31. [PubMed]
- Remmers JE, deGroot WJ, Sauerland EK, et al. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 1978;44:931-8. [PubMed]
- Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am J Respir Crit Care Med 1996;153:1880-7. [PubMed]
- Schwartz AR, O’Donnell CP, Baron J, et al. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am J Respir Crit Care Med 1998;157:1051-7. [PubMed]
- Steier J, Jolley CJ, Seymour J, et al. Increased load on the respiratory muscles in obstructive sleep apnea. Respir Physiol Neurobiol 2010;171:54-60. [PubMed]
- Oliven A, Odeh M, Gavriely N. Effect of hypercapnia on upper airway resistance and collapsibility in anesthetized dogs. Respir Physiol 1989;75:29-38. [PubMed]
- Oliven A, Odeh M, Gavriely N. Effect of salicylate on upper airway dilating muscles in anesthetized dogs. Am Rev Respir Dis 1989;139:170-5. [PubMed]
- Van de Graaff WB, Gottfried SB, Mitra J, et al. Respiratory function of hyoid muscles and hyoid arch. J Appl Physiol 1984;57:197-204. [PubMed]
- Miki H, Hida W, Chonan T, et al. Effects of submental electrical stimulation during sleep on upper airway patency in patients with obstructive sleep apnea. Am Rev Respir Dis 1989;140:1285-9. [PubMed]
- Edmonds LC, Daniels BK, Stanson AW, et al. The effects of transcutaneous electrical stimulation during wakefulness and sleep in patients with obstructive sleep apnea. Am Rev Respir Dis 1992;146:1030-6. [PubMed]
- Decker MJ, Haaga J, Arnold JL, et al. Functional electrical stimulation and respiration during sleep. J Appl Physiol (1985) 1993;75:1053-61. [PubMed]
- Hida W, Okabe S, Miki H, et al. Effects of submental stimulation for several consecutive nights in patients with obstructive sleep apnoea. Thorax 1994;49:446-52. [PubMed]
- Guilleminault C, Powell N, Bowman B, et al. The effect of electrical stimulation on obstructive sleep apnea syndrome. Chest 1995;107:67-73. [PubMed]
- Hu L, Xu X, Gong Y, et al. Percutaneous biphasic electrical stimulation for treatment of obstructive sleep apnea syndrome. IEEE Trans Biomed Eng 2008;55:181-7. [PubMed]
- Steier J, Seymour J, Rafferty GF, et al. Continuous transcutaneous submental electrical stimulation in obstructive sleep apnea: a feasibility study. Chest 2011;140:998-1007. [PubMed]
- Oliven A, Odeh M, Schnall RP. Improved upper airway patency elicited by electrical stimulation of the hypoglossus nerves. Respiration 1996;63:213-6. [PubMed]
- Fuller D, Mateika JH, Fregosi RF. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. J Physiol 1998;507:265-76. [PubMed]
- Eisele DW, Smith PL, Alam DS, et al. Direct hypoglossal nerve stimulation in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 1997;123:57-61. [PubMed]
- Bishara H, Odeh M, Schnall RP, et al. Electrically-activated dilator muscles reduce pharyngeal resistance in anaesthetized dogs with upper airway obstruction. Eur Respir J 1995;8:1537-42. [PubMed]
- Schwartz AR, Eisele DW, Hari A, et al. Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol (1985) 1996;81:643-52. [PubMed]
- Eisele DW, Schwartz AR, Smith PL. Tongue neuromuscular and direct hypoglossal nerve stimulation for obstructive sleep apnea. Otolaryngol Clin North Am 2003;36:501-10. [PubMed]
- Schwartz AR, Thut DC, Russ B, et al. Effect of electrical stimulation of the hypoglossal nerve on airflow mechanics in the isolated upper airway. Am Rev Respir Dis 1993;147:1144-50. [PubMed]
- Mwenge GB, Rombaux P, Dury M, et al. Targeted hypoglossal neurostimulation for obstructive sleep apnoea: a 1-year pilot study. Eur Respir J 2013;41:360-7. [PubMed]
- Schwartz AR, Barnes M, Hillman D, et al. Acute upper airway responses to hypoglossal nerve stimulation during sleep in obstructive sleep apnea. Am J Respir Crit Care Med 2012;185:420-6. [PubMed]
- Eastwood PR, Barnes M, Walsh JH, et al. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep 2011;34:1479-86. [PubMed]
- Schwartz AR, Bennett ML, Smith PL, et al. Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 2001;127:1216-23. [PubMed]
- Van de Heyning PH, Badr MS, Baskin JZ, et al. Implanted upper airway stimulation device for obstructive sleep apnea. Laryngoscope 2012;122:1626-33. [PubMed]
- Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014;370:139-49. [PubMed]
- Sullivan CE, Issa FG, Berthon-Jones M, et al. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1981;1:862-5. [PubMed]
- McArdle N, Devereux G, Heidarnejad H, et al. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 1999;159:1108-14. [PubMed]
- World Health Organisation (WHO), Global status report on noncommunicable diseases 2010. Available online: http://www.who.int/nmh/publications/ncd_report2010/en/
- Rodenstein D, Rombaux P, Lengele B, et al. Residual effect of THN hypoglossal stimulation in obstructive sleep apnea: a disease-modifying therapy. Am J Respir Crit Care Med 2013;187:1276-8. [PubMed]
- Oliven A, Odeh M, Geitini L, et al. Effect of coactivation of tongue protrusor and retractor muscles on pharyngeal lumen and airflow in sleep apnea patients. J Appl Physiol (1985) 2007;103:1662-8. [PubMed]
- Schwartz AR, Schubert N, Rothman W, et al. Effect of uvulopalatopharyngoplasty on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis 1992;145:527-32. [PubMed]
- Vanderveken OM, Maurer JT, Hohenhorst W, et al. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med 2013;9:433-8. [PubMed]
- Randerath WJ, Verbraecken J, Andreas S, et al. Non-CPAP therapies in obstructive sleep apnoea. Eur Respir J 2011;37:1000-28. [PubMed]
- Theurel J, Lepers R, Pardon L, et al. Differences in cardiorespiratory and neuromuscular responses between voluntary and stimulated contractions of the quadriceps femoris muscle. Respir Physiol Neurobiol 2007;157:341-7. [PubMed]
- Edwards RH, Young A, Hosking GP, et al. Human skeletal muscle function: description of tests and normal values. Clin Sci Mol Med 1977;52:283-90. [PubMed]
- Malhotra A, Huang Y, Fogel R, et al. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. Am J Med 2006;119:72.e9-14.
- Schwartz AR, Gold AR, Schubert N, et al. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis 1991;144:494-8. [PubMed]
- Eisele DW, Schwartz AR, Hari A, et al. The effects of selective nerve stimulation on upper airway airflow mechanics. Arch Otolaryngol Head Neck Surg 1995;121:1361-4. [PubMed]
- Dotan Y, Golibroda T, Oliven R, et al. Parameters affecting pharyngeal response to genioglossus stimulation in sleep apnoea. Eur Respir J 2011;38:338-47. [PubMed]
- Maurer JT, Van de Heyning P, Lin HS, et al. Operative technique of upper airway stimulation: an implantable treatment of obstructive sleep apnea. Oper Tech Otolaryngol Head Neck Surg 2012;23:227-33.


