CT findings in pulmonary alveolar proteinosis: serial changes and prognostic implications
Introduction
Pulmonary alveolar proteinosis (PAP) is a rare disease of unknown etiology that is characterized by impaired surfactant metabolism, which leads to the accumulation of proteinaceous periodic acid—Schiff-positive material in the alveolar space (1,2). Patients with PAP generally present with subacute indolent symptoms, including progressive dyspnea and cough (3,4).
PAP-associated imaging findings are well-characterized. Radiographs typically reveal bilateral symmetric and central opacities with a perihilar or basal distribution. High-resolution CT (HRCT) scans show smooth interlobular or intralobular septal thickening superimposed on a background of ground-glass opacities (GGOs), resulting in a “crazy-paving” appearance (1,5,6).
The survival rate associated with PAP is relatively high as a result of the use of whole-lung lavage (WLL). Prior to the widespread use of WLL, which began in 1960, the mortality rate was much higher (approximately 30%); currently, the 5-year survival rate of patients who undergo WLL is 95% (3,7). Recently, exogenous granulocyte macrophage colony-stimulating factor (GM-CSF) has been used to treat patients with PAP by alleviating GM-CSF deficiency. However, the natural history of PAP is not well-defined. Despite recent advances in the treatment of patients with PAP, some patients experience residual disease after treatment; others exhibit progression to end-stage pulmonary fibrosis, which necessitates lung transplantation (3,8). Thus, additional studies are needed to accurately ascertain the clinical outcomes of patients with PAP, as well as factors that can be used to predict patient prognosis. To our knowledge, the prognostic implications of serial CT findings for patients with PAP have not yet been evaluated. Therefore, this study aimed to characterize serial CT findings for patients with PAP, and to identify factors that are predictive of clinical improvement.
Methods
Patient enrollment and demographics
This retrospective study was approved by the institutional review board of Samsung Medical Center in South Korea; requirement for patient informed consent was waived because of the retrospective nature of the study. Twenty-five patients with PAP were identified who had pathologically proven diagnoses as a result of positive surgical biopsies (n=10), transbronchial lung biopsies (n=6), or cytological analyses of bronchoalveolar lavage fluid (n=9), during the period between September 1997 and January 2015. Patients with opportunistic infections were excluded. The enrolled patients consisted of 16 men and nine women, with a mean age of 47 years (range: 15–77 years). Medical records of all enrolled patients were reviewed for clinical features (e.g., symptoms), laboratory findings, treatment, and radiological findings. A clinician (MP Chung) classified the patients into two groups: those with clinical improvement and those with progression or no change at final follow-up, primarily based on patient signs and symptoms, such as dyspnea, cough, sputum, and crackles on auscultation.
Pulmonary function tests (PFTs)
Data from PFTs at the time of PAP diagnosis and final follow-up were obtained for 20 patients. Spirometric measurements [i.e., forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), the ratio between forced expiratory volume in 1 second and forced vital capacity (FEV1/FVC)], and single-breath diffusing capacity of the lung for carbon monoxide (DLCO) were obtained by using pulmonary function units (SensorMedics Corp., Yorba Linda, CA, USA). FVC and DLCO values were each expressed as a percentage of the predicted value, which was based on patient height, age, sex, and ethnicity. Additionally, the partial pressure of arterial oxygen (PaO2; when breathing room air) was measured concurrent with the performance of PFTs.
Image acquisition
All patients underwent 131 HRCT scans (mean: 5.2±3.9 CT examinations per patient; range, 2–21), which began at baseline and continued until the final follow-up scans. The scanning parameters were 120 kVp and 90–170 mA, respectively. Helical CT scans (beam width: 10–20 mm; beam pitch: 1.375–1.500) of the thorax were obtained; the scan data were reconstructed at 10-mm intervals with 1.00–1.25-mm section thickness, covering the apex to the base of each lung. The CT data were reconstructed by using a bone algorithm; the imaging data were displayed directly on four monitors (each with 1,536×2,048 image matrices, 8-bit viewable grayscale, and 60-foot Lambert luminescence) of a Centricity Picture Archiving and Communication System 4.0 (GE Healthcare, Mt. Prospect, IL, USA). Both mediastinal (window width: 400 HU; window level: 20 HU) and lung (window width: 1,500 HU; window level: −700 HU) window images were available for analysis.
Analysis of HRCT findings
The CT scans were independently reviewed by two radiologists (BD Nam and TJ Kim, who had 5 and 18 years of experience of thoracic CT scan interpretation, respectively); final decisions were reached by consensus. The radiologists assessed the patterns (i.e., areas of GGO, interlobular or intralobular septal thickening, consolidation, crazy-paving appearance, airway irregularity, traction bronchiectasis, architectural distortion, and honeycombing) by using definitions published in the literature (9). The extent (to the nearest 5%), and the distribution (central, peripheral, or diffuse axial distribution, and upper, lower, or diffuse vertical distribution) of lung abnormalities were also analyzed. The overall extent of lung abnormalities and changes therein were evaluated by comparing baseline and final follow-up CT scans. Changes in the overall extent of lung abnormalities were divided into the following categories: complete resolution, improvement (>10% decrease), no change (≤10% change), and progression (>10% increase). CT patterns were compared between patients with idiopathic and secondary PAP; furthermore, CT patterns of patients who exhibited clinical improvement after WLL were compared with those of patients who did not exhibit clinical improvement after WLL.
Statistical analyses
Statistical analyses were conducted by using SPSS version 18.0 (SPSS, Chicago, IL, USA) and MedCalc version 9.0 (MedCalc Software, Mariakerke, Belgium). The clinical features, baseline and final follow-up CT findings, and PFT results were compared between patients who exhibited clinical improvement and those who exhibited progression or no change. Categorical variables were compared by using Fisher’s exact test; continuous variables were compared by using the Mann-Whitney U test. Univariate and multivariate analyses were conducted to identify clinical or CT features that were predictive of clinical improvement in PAP. Variables with P value <0.1 in univariate analyses were included in multivariate analyses. Intraclass correlation coefficients (ICCs) were calculated to assess inter-reader agreement regarding the extent of each type of lung abnormality, overall extent of lung abnormalities, and changes therein. In all statistical analyses, P values <0.05 were considered statistically significant.
Results
Patient enrollment, demographics, and clinical findings
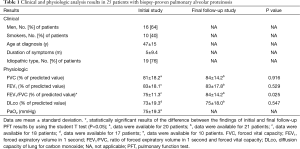
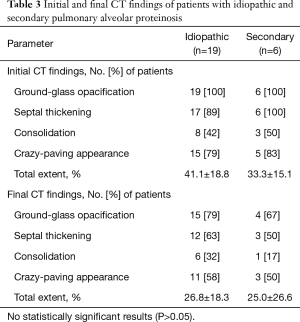
The demographics and clinical findings of 25 patients with pathologically proven PAP are summarized in Table 1. Twenty (80%) of the 25 patients presented with symptoms at the time of diagnosis; most had cough (n=19, 76%) and dyspnea (n=16, 64%). The mean period of clinical onset was 5 months (range, 1–36 months). Regarding the patients’ smoking statuses, 15 were nonsmokers, 10 were ex-smokers (mean duration: 27 pack-years), and none were current smokers. Idiopathic PAP was observed in 19 patients and secondary PAP was observed in six. One patient with secondary PAP exhibited acute lymphoblastic leukemia. Three patients reported a history of inhalation exposure to toxic fumes (welding) and two reported a history of exposure to non-organic dusts (masonry). Serum GM-CSF autoantibody tests were not performed.
Full table
Nine (36%) patients underwent WLL, with a mean of 2.3±1.8 treatments (range, 1–6). Sixteen (64%) patients did not undergo treatment, such as GM-CFS therapy, plasmapheresis, or lung transplantation. At final follow-up, the symptoms at initial presentation were completely resolved or improved in 10 (40%) patients, whereas they were unchanged or showed progression in 15 (60%). Seven (70%) of the 10 improved patients had experienced spontaneous remission; the other three (30%) underwent, and responded to, WLL.
PFT results
The PFT results are summarized in Table 1. Although the mean FVC, FEV1, FEV1/FVC, and DLco values at final follow-up were consistently higher than those at initial diagnosis, only the change in FEV1/FVC was statistically significant.
Patients with clinical improvement at final follow-up also tended to demonstrate better PFT results (e.g., FVC, FEV1, FEV1/FVC, and DLco values), compared with patients who showed progression or no change. However, only changes in FVC and DLco at the final follow-up significantly differed between the two groups: 91.4%±7.1% versus 79.2%±14.4% in FVC, P=0.047; 87.1%±19.2% versus 67.3%±12.0% in DLco, P=0.007.
Serial HRCT findings
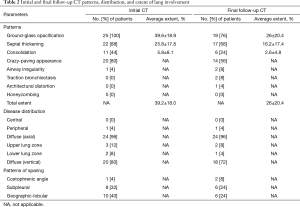
Serial HRCT scans were obtained over a mean follow-up period of 39±24 months (median, 38 months; range, 2–96 months). The mean number of follow-up studies was 3.9 (range, 2–21 studies). Table 2 summarizes the CT patterns, distribution, and extent of lung abnormalities in baseline and final follow-up HRCT scans. The most common patterns of lung abnormalities on baseline CT scans were GGO (n=25, 100%) and septal thickening (n=22, 88%), followed by crazy-paving appearance (n=20, 80%). The distribution of lung abnormalities was typically diffuse, with no tendency towards central or peripheral distributions, or towards upper or lower distributions. The patterns of sparing of parenchymal opacification included geographic-lobular (n=10, 40%), subpleural region (n=8, 32%), and costophrenic angle (n=1, 4%) patterns, with some overlapping between patterns.
Full table
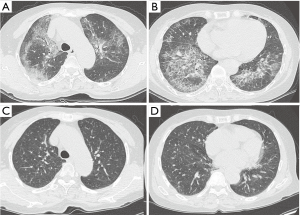
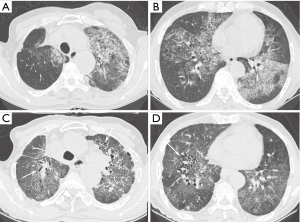
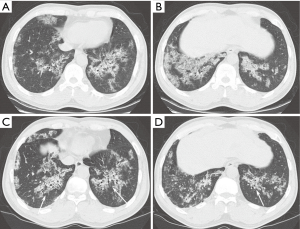
According to the final follow-up CT scans, 13 (52%) patients exhibited improvement in lung abnormalities, including five patients with complete resolution (Figure 1); 12 (48%) patients showed progression (n=3) or no change (n=9). The most common CT findings on the final follow-up scans were GGO (n=19, 76%) septal thickening (n=16, 64%), and crazy-paving appearance (n=14, 56%). CT features suggestive of pulmonary fibrosis (i.e., traction bronchiectasis) were observed for two (8%) patients; one (4%) also exhibited architectural distortion (Figures 2 and 3). None of the baseline or final follow-up CT scans showed honeycombing.
No significant differences were found in CT patterns between idiopathic and secondary PAP patients (Table 3); furthermore, there were no significant differences in CT features between patients with clinical improvement (n=3) and those without clinical improvement (n=6) among patients who underwent WLL. Inter-reader agreement regarding the extent of each type of lung abnormality was excellent (ICCs for GGO: 0.98; consolidation: 0.89; septal thickening: 0.98; overall extent: 0.98; change in overall extent: 0.98).
Full table
Correlations between clinical findings and CT and PFT results
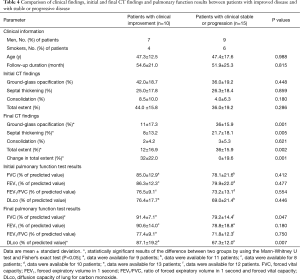
The clinical features, baseline and final CT findings, and PFT results were compared between patients with clinical improvement (n=10) and those with progression or no change (n=15), by using univariate analysis (Table 4). There was a significant difference between the two groups with respect to the extent of GGO on the final CT scans. The group that showed progression or no change had a greater extent of GGO on the final CT scans. In addition, both the overall extent of lung abnormalities on the final CT scan and change in overall extent for patients with progression or no change were significantly higher than for patients with clinical improvement. There were also significant differences in FVC and DLco between the two groups; the group that experienced clinical improvement demonstrated better lung function than the group with progression or no change.
Full table
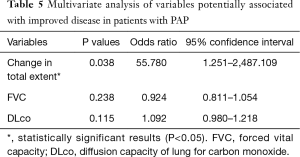
Multivariate analysis showed that change in the overall extent of lung abnormalities on the final follow-up CT scan independently predicted clinical improvement in patients with PAP (OR: 55.780; 95% confidence interval: 1.251–2487.109; P=0.038) (Table 5).
Full table
Discussion
In this cohort study, we evaluated serial HRCT findings in patients with pathologically proven PAP who were treated at a single tertiary referral hospital; moreover, we identified CT features that were predictive of clinical improvement at long-term follow-up. Most patients (80%) showed residual disease, according to the final follow-up HRCT scans; however, progression to pulmonary fibrosis was rare (8%). Change in the overall extent of lung abnormalities on serial CT scans appeared to be predictive of clinical improvement in PAP.
The most common finding of the baseline CT scans was GGO (100%), followed by interlobular or intralobular septal thickening (88%), which is consistent with the findings of previous studies (1,2,5,10). The distribution of lung abnormalities was diffuse (96%) with no zonal predominance; this also agrees with the findings of previous studies (1,5,11). However, geographic or patchy distributions, with either central or peripheral lung involvement, have been reported in the literature (12,13). In this study, sharply marginated areas of geographic or lobular and subpleural sparing were frequently apparent on baseline CT scans; notably, this is consistent with the findings of previous studies (1,12,13). Although we did not evaluate the relationships between CT and PFT findings, it has been suggested that CT findings (e.g., HRCT scan severity) in patients with PAP are closely related to PFT results (e.g., DLco, FEV1/FVC, and PaO2) (2).
The most common lung abnormality on the final follow-up CT scans was GGO (76%), followed by septal thickening (68%); this was similar to the abnormalities that were associated with the baseline CT scans. The mean extent of lung abnormalities was reduced on the final follow-up CT scans; however, regardless of the treatment administered, there were differences between patients with respect to changes in lung abnormalities; namely, 20% experienced complete resolution, 32% experienced improvement, 36% experienced no change, and 12% experienced progression. Holbert et al. (1), in a study of 20 patients with PAP, showed that there were no consistent trends in the type or distribution of opacities on follow-up CT scans. Thus far, few studies have investigated serial CT findings in patients with PAP, and little is known regarding the association between follow-up CT findings and clinical outcomes. In this study, only two (8%) patients had CT features suggestive of pulmonary fibrosis (e.g., airway irregularity, traction bronchiectasis, and architectural distortion). The first of the two patients with traction bronchiectasis did not exhibit underlying interstitial pneumonia or fibrosis; however, the patient experienced recurrent episodes of PAP and underwent four WLL treatments. Serial follow-up CT scans (n=9) over 6 years showed that the patient exhibited GGO, which evolved into traction bronchiectasis and architectural distortion with a mild reduction in lung volume; this represented progression to pulmonary fibrosis (Figure 2). The serial CT findings of the other patient with traction bronchiectasis showed a gradual reduction in the areas of GGO; however, the bronchial dilatation increased slightly (Figure 3). These findings suggest that a small subset of patients with PAP may experience progression to pulmonary fibrosis; however, patients with PAP generally demonstrate a good prognosis.
Although the term “pulmonary alveolar proteinosis” implies alveolar disease, PAP-associated CT patterns can range from alveolar air space disease to interstitial disease. PAP involving interstitial manifestations is typically mild. However, a small number of cases of significant pulmonary fibrosis have been reported in association with PAP (10,14-17). For example, Agarwal et al. (14) reported that a patient with PAP exhibited a central distribution of fibrosis according to HRCT scans; this was an important finding in the exclusion of the coexistence of idiopathic pulmonary interstitial fibrosis. This finding is consistent with our results. The CT findings that were suggestive of pulmonary fibrosis in the two patients in our study indicated diffuse but relatively central distributions, rather than peripheral distributions. In three previous studies regarding PAP with pulmonary fibrosis (15-17), it was difficult to differentiate between coexistent fibrosis and the progression of PAP to fibrosis; therefore, surgical lung biopsies were necessary. In our study, pulmonary fibrosis was confirmed in one patient who underwent surgical lung biopsy. Our finding regarding the rarity of the progression of PAP to fibrosis concurs with the results of the study by Holbert et al. (1), which found that among 27 patients with PAP, only two (7%) showed substantial fibrosis and eight (30%) showed mild, clinically insignificant fibrosis. A recent study of 44 PAP patients reported a relatively high incidence of pulmonary fibrosis (20%) with poor prognosis. The higher incidence might have been caused by the inclusion of patients with more severe cases of PAP in the multicenter study setting (10). Future studies in a larger cohort are required to confirm the association of PAP with pulmonary fibrosis and patient prognosis.
The natural history of PAP varies among patients, regardless of treatment. In the present study, 24% of patients with PAP experienced spontaneous remission without treatment; 40% of patients who experienced clinical improvement in PAP had undergone WLL. Similarly, Kariman et al. (18) performed a study of 23 patients with PAP; they reported that 24% of patients showed clinical improvement without treatment, and that 79% of patients who were treated with WLL responded to the treatment. In our study, both patients who showed progression to pulmonary fibrosis had undergone WLL.
To the best of our knowledge, there have been no previous reports regarding features of CT scans that can be used to predict clinical improvement or recurrence of PAP. In the present study, univariate analyses showed that the extent of GGO, extent of septal thickening, overall extent of lung abnormalities, and changes in the overall extent between the baseline and final CT scans were significantly associated with clinical improvement. Change in the overall extent of lung abnormalities also constituted a significant predictor of clinical improvement in the multivariate analysis. Notably, this is the first study to demonstrate that features of serial CT scans may predict prognosis in patients with PAP. However, further studies with large patient cohorts are needed to confirm whether features of CT scans can predict clinical outcomes in patients with PAP.
This study had several limitations. First, the study design was retrospective and some data regarding clinical findings and PFT results were missing. Second, the sample size was small, although we included as many patients as possible from among patients with pathologically proven PAP (i.e., all patients for whom serial CT scans were available). In addition, we compared the frequency of CT features between idiopathic and secondary PAP, but could not perform additional quantitative analysis due to the small number of patients in each group. Third, multivariate analysis indicated that the confidence interval for the change in the overall extent of lung abnormalities was large, which might be a result of the small sample size. Further studies with large sample sizes are required to validate independent predictors of clinical outcomes in patients with PAP. Fourth, the follow-up periods varied; the shortest follow-up period comprised 2 months (median: 38 months; range: 2–96 months). Fifth, detailed clinical histories and laboratory results, such as GM-CSF autoantibodies, could not be obtained due to the retrospective design of the study. In conclusion, serial CT scans in patients with PAP indicated residual disease in most patients; however, progression to pulmonary fibrosis was rare. Change in the overall extent of lung abnormalities on serial CT scans appears to be predictive of clinical improvement in PAP.
Acknowledgements
Funding: This study was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health and Welfare (1520230), Republic of Korea.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional review board of Samsung Medical Center in South Korea and the requirement for written informed consent was waived by the Institutional Review Board.
References
- Holbert JM, Costello P, Li W, et al. CT features of pulmonary alveolar proteinosis. AJR Am J Roentgenol 2001;176:1287-94. [Crossref] [PubMed]
- Lee KN, Levin DL, Webb WR, et al. Pulmonary alveolar proteinosis: High-resolution CT, chest radiographic, and functional correlations. Chest 1997;111:989-95. [Crossref] [PubMed]
- Ioachimescu OC, Kavuru MS. Pulmonary alveolar proteinosis. Chron Respir Dis 2006;3:149-59. [Crossref] [PubMed]
- Presneill JJ, Nakata K, Inoue Y, et al. Pulmonary alveolar proteinosis. Clin Chest Med 2004;25:593-613. viii. [Crossref] [PubMed]
- Frazier AA, Franks TJ, Cooke EO, et al. From the archives of the AFIP: pulmonary alveolar proteinosis. Radiographics 2008;28:883-99. [Crossref] [PubMed]
- Johkoh T, Itoh H, Muller NL, et al. Crazy-paving appearance at thin-section CT: spectrum of disease and pathologic findings. Radiology 1999;211:155-60. [Crossref] [PubMed]
- Michaud G, Reddy C, Ernst A. Whole-lung lavage for pulmonary alveolar proteinosis. Chest 2009;136:1678-81. [Crossref] [PubMed]
- Parker LA, Novotny DB. Recurrent alveolar proteinosis following double lung transplantation. Chest 1997;111:1457-8. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Akira M, Inoue Y, Arai T, et al. Pulmonary Fibrosis on High-Resolution CT of Patients With Pulmonary Alveolar Proteinosis. AJR Am J Roentgenol 2016;207:544-51. [Crossref] [PubMed]
- Shah PL, Hansell D, Lawson PR, et al. Pulmonary alveolar proteinosis: clinical aspects and current concepts on pathogenesis. Thorax 2000;55:67-77. [Crossref] [PubMed]
- Godwin JD, Muller NL, Takasugi JE. Pulmonary alveolar proteinosis: CT findings. Radiology 1988;169:609-13. [Crossref] [PubMed]
- Newell JD, Underwood GH Jr, Russo DJ, et al. Computed tomographic appearance of pulmonary alveolar proteinosis in adults. J Comput Tomogr 1984;8:21-9. [Crossref] [PubMed]
- Agarwal PP, Seely JM, Perkins DG, et al. Pulmonary alveolar proteinosis and end-stage pulmonary fibrosis: a rare association. J Thorac Imaging 2005;20:242-4. [Crossref] [PubMed]
- Arbiser ZK, Guidot DM, Pine JR, et al. Pulmonary alveolar proteinosis mimicking idiopathic pulmonary fibrosis. Ann Diagn Pathol 2003;7:82-6. [Crossref] [PubMed]
- Clague HW, Wallace AC, Morgan WK. Pulmonary interstitial fibrosis associated with alveolar proteinosis. Thorax 1983;38:865-6. [Crossref] [PubMed]
- Miller PA, Ravin CE, Smith GJ, et al. Pulmonary alveolar proteinosis with interstitial involvement. AJR Am J Roentgenol 1981;137:1069-71. [Crossref] [PubMed]
- Kariman K, Kylstra JA, Spock A. Pulmonary alveolar proteinosis: prospective clinical experience in 23 patients for 15 years. Lung 1984;162:223-31. [Crossref] [PubMed]


