EGFR immunoexpression, RAS immunoexpression and their effects on survival in lung adenocarcinoma cases
Introduction
Epidermal growth factor receptor (EGFR), also known as ErbB1 is an intracellular transmembrane glycoprotein, which has intrinsic tyrosine kinase activity (1,2). When the ligand is bound to the cell, autophosphorylation occurs in the intracytoplasmic segment, which activates intracellular tyrosine kinase (1). As a consequence RAS-RAF-MAPK (mitogen activated protein kinase) signal transduction pathway is activated (3). EGFR overexpression causes tumor cell growth, tumor invasion, angiogenesis and eventually metastasis (2). A relation between EGFR overexpression and cellular adhesion, inhibition of apoptosis and resistance to chemotherapy have been identified (4). EGFR gene mutations have been confirmed to play role in development of pulmonary adenocarcinoma (5). EGFR expression can be detected in non-small cell lung cancer (NSCLC) cases with immunohistochemical staining (6). EGFR overexpression has been shown to have negative impacts on prognosis in NSCLC patients by a recent meta-analysis (2,7,8).
RAS is the human analog of a gene, which is coded by a retrovirus and causes sarcoma in rats (9). RAS is activated by binding guanosine triphosphate and facilitates intracellular signal transduction (10). K-RAS gene codon 12 mutation has been identified in approximately 40% of lung adenocarcinoma cases. RAS mutation plays an important role in cell growth and inhibition of apoptosis (11). RAS can be detected with immunohistochemical techniques in NSCLC cases (12). A meta-analysis has suggested that RAS has no effect on prognosis in NSCLC cases (13).
This study aims at identifying the presence of EGFR immunoexpression and RAS immunoexpression and their effects on survival in pulmonary adenocarcinoma cases.
Materials and methods
Patients
Twenty-six patients, who underwent complete anatomical resection and mediastinal lymph node dissection due to bronchial adenocarcinoma at Hacettepe University Hospital, Department of Thoracic Surgery between 2002 and 2007, were included in the study. The study was conducted upon approval of local ethics committee of our university. The preoperative diagnostic and metastatic workup was carried out for each patient and the resectable cases were operated on. At least one year follow up survival data was recorded for all patients. Stage of the disesase, lymph node involvement, lymphovascular invasion, and pleural invasion were noted for each patient according to the pathological examination reports after the surgery. EGFR and RAS immunoexpressions were examined using the paraffin blocks of the pathological specimens.
Immunohistochemistry
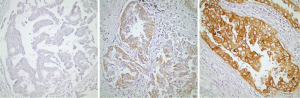
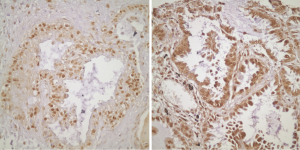
Twenty of the patients that are included in the study had tissue microarray (TMA) of their pathological specimens. TMA of the 20 patients and the routine paraffin blocks of the remaining six patients were used for the preparation of 5 µm slides. Antigen retrieval procedure and avidin-biotin-peroxidase method were used for all prepared sections. 21E1-1 mouse, monoclonal antibody (ImmunoVision Technologies, USA) and RB-1627P rabbit, polyclonal antibody (Neomarkers, Fremont, CA, USA) were used respectively for EGFR and RAS. Immunohistochemical results were evaluated by using Zeiss Axioskop 2. For EGFR, the intensity of the staining was grouped as: absent (score 0), weak (score 1), medium (score 2) and strong (score 3). The percentage of the staining was grouped as: 0-10% (score 1), 11-50% (score 2) and 51-100% (score 3). The degree of immunoexpression was categorized by the multiplication of intensity score by the percentage score. A multiplication score of 0 was considered to be negative; 1, 2 and 3 were considered to be weak; and 4 and 6 were considered to be strong expressions for EGFR (Figure 1). The sections were grouped as weak and strong expressions according to the nuclear staining patterns of RAS (Figure 2).
Statistical analysis
Since the number of the patients included in the study was less than 30, nonparametric analyses were used. The relationships of EGFR immunoexpression and RAS immunoexpression with survival, stage of the disease, lymphovascular invasion, and pleural invasion were analyzed using Kendall’s tau_b ve Spearman’s rho tests in the SPSS 11.5 program.
Results
Twenty-six patients included in the study consisted of 18 (69.2%) male and 8 (30.8%) female participants. The mean age of patients was 56.3±10.4 [35-74]. At the time of the study, 18 (69.2%) patients were alive and 8 (30.8%) had passed away. According to the staging conducted after the pathological examination of the resected specimens; 6 (23.1%) patients were Stage IA, 10 (30.8%) patients were Stage IB, 1 (3.8%) patient was Stage IIA, 1 (3.8%) patient was Stage IIB, 7 (26.9%) patients were Stage IIIA and 1 (3.8%) patient was Stage IIIB. Seventeen (65.4%) patients had N0, 1 (3.8%) patient had N1 and 8 (30.8%) patients had N2 disease after the tissue evaluation of the surgically dissected lymph nodes. Seventeen (65.4%) patients had lymphovascular invasion and 9 (34.6%) patients did not have lymphovascular invasion. Twelve (46.2%) patients had pleural invasion, while 14 (53.8%) patients did not.
EGFR immunoexpression was negative for 12 (46.2%) patients, weak for 9 (34.6%) patients and strong for 5 (19.2%) patients. RAS immunoexpression was weak for 6 (23.1%) patients and strong for 20 (76.9%) patients. EGFR immunoexpression, RAS immunoexpression and the clinicopathological parameters of the patients are shown in Table 1.

Full table
Nonparametric bivariate analyses were used for the evaluation of the statistically significant relationship between the immunoexpression status and survival, stage of the disease, nodal involvement, lymphovascular invasion; and pleural invasion. Strong EGFR immunoexpression had a negative relationship with survival, which is statistically significant at α =0.05 level according to Kendall’s tau_b and Spearman’s rho tests (Kendall’s tau_b, r =–0.400; Spearman’s rho, r =–0.421; Table 2). There was no statistically significant relationship between EGFR immunoexpression and stage of the disease, nodal involvement, lymphovascular invasion, or pleural invasion (Kendall’s tau_b, r =–0.075, –0.032, –0.271, –0.037; Spearman’s rho, r =–0.088, –0.042, –0.286, –0.039, respectively). RAS immunoexpession had no significant relationship with survival, stage of the disease, nodal involvement, lymphovascular invasion, or pleural invasion. (Kendall’s tau_b, r =–0.167, –0.134, –0.194, –0.177, –0.225; Spearman’s rho, r =–0.167, –0.147, –0.197, –0.177, –0.225, respectively) (Table 3).
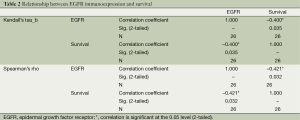
Full table
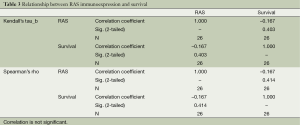
Full table
Discussion
Lung cancer is one of the leading causes of death in developed countries. The poor prognosis in lung carcinoma may be due to the patient related factors or the influences associated with the self-nature of the tumor (8). Age, patient status, stage of the disease are among the survival predictors in resectable NSCLC (14). Serum lactate dehydrogenase level, white cell count and neutrophil count are also found to be effective on survival (15). Recently, new techniques to investigate prognosis have been put forward with the advances in molecular biology and cytogenetics (8).
The development and progression of cancer is caused by various factors on the cellular level, such as autonomous growth signals, refraction to growth inhibiting signals, insensitivity to apoptotic signals, unlimited growth potential, angiogenesis, invasion and metastasis (16). Several proto-oncogenes and tumor suppressor genes play a role in these genetic irregularities (17). Some of these genetic irregularities affect the tumor cell behavior much more, and thus can be used as prognostic markers. Molecular prognostic markers reveal themselves as changes in gene copy number, messenger ribonucleic acid (mRNA), and expression levels of proteins (12).
Immunohistochemistry is a quite practical method for detecting the changes in protein expression. It not only shows protein expression in a semi-quantitative manner, but also provides information on the cellular localization of the protein expression. Immunohistochemistry has been used in many different studies as it is involved in literature in this field (12).
EGFR, which has intrinsic tyrosine kinase activity, is a transmembrane protein with an intracellular domain (1). EGFR activates a couple of intracellular signal transduction pathways (7). These pathways cause the cells to transform and grow, inhibit apoptotic signals, and lead to angiogenesis and tumor invasion (18). Kozuki et al. have shown mutations of the EGFR gene in the development of lung adenocarcinoma (5).
The effect of EGFR expression on survival in NSCLC has been studied by immunohistochemical methods and different results were obtained. In 1997, Rusch et al. found EGFR expression to have an effect on survival of NSCLC by using Northern Blot and immunohistochemical methods (19). Volm et al. stated that EGFR immunoexpression has a negative effect on survival of squamous cell lung cancer cases (20). In 2000, by conducting an immunohistochemical study, Ohsaki et al. have similarly found a negative effect of EGFR expression on survival of NSCLC patients (21). Meert et al. conducted a meta-analysis in 2002 by reviewing 16 different studies, which were published between 1989 and 2000. Fourteen of these studies were based on immunohistochemical methods. EGFR expression was found to be positive in 51% NSCLC cases and 46.2% lung adenocarcinoma cases. In a quantitative meta-analysis of eight studies, EGFR expression was shown to be a poor prognostic sign (8). In the current study, the researchers examined the pathological specimens of the patients who were operated for lung adenocarcinoma and grouped them as negative, weak, and strong according to the immunohistochemically detected EGFR expression. Since the total number of the patients was less than 30, the researchers used nonparametric bivariate analyses for the statistical evaluation. Strong EGFR immunoexpression was found to affect survival in a negative fashion. This result is consistent with that obtained by Meert et al. in their meta-analysis. In another meta-analysis carried out by Nicholson et al. EGFR expression was found to negatively affect the survival in 10-20% of the studies they reviewed (22). In 2005, Niemiec et al. showed a statistically significant relationship between EGFR and prognosis (23). The results of the current study are parallel to some of those in the literature. The different results achieved in various studies are dependent on the immunohistochemical methods used. There is no standard evaluation method for EGFR immunoexpression. The scoring is semi-quantitative. The scoring methods depend on the evaluation of staining intensity, percentage of the stained cells, the location of the staining, and the combination of these three parameters (12). The combination process of the staining intensity and percentage of stained cells also varies in different studies. These factors lead to different immunoexpression levels.
Lung adenocarcinoma cases have been shown to bear 40% K-RAS gene codon 12 mutation (11). RAS proteins bind GTP. They play a key role in intracellular molecular events. The intrinsic GTPase activity of RAS is lost due to the mutations in tumor cells, and the intracellular signal transduction pathways are continuously stimulated. This gives rise to uncontrolled cell proliferation (13). RAS mutation is important in cell growth and the inhibition of apoptosis (11). The relationship of RAS with prognosis of the disease has been evaluated in various studies using immunohistochemical methods. In NSCLC patients, Harada et al. showed RAS to be important for survival, to be an independent prognostic factor, and to be a good marker for understanding the malignant potential of the tumor (24). Miyamato et al. used immunohistochemical methods and found RAS expression to be a survival predictor independent of tumor stage (25). In 2005 Mascaux et al. published a meta-analysis in which they evaluated the relationship between RAS expression and survival in lung cancer patients (13). Both immunohistochemistry and other molecular diagnostic methods were used in the studies included in this meta-analysis. In a couple of these studies, there was a statistically significant negative relationship between RAS expression and survival. Yet in a considerable amount of them, there was no relationship. There were also some papers denoting that RAS expression an effect on the metastatic potential of the tumor. The current study did not find a significant relationship between RAS expression and survival. These results are consistent with some of the literature. The studies conducted on a larger number of patients with the use of molecular diagnostic techniques may lead to better results for the evaluation of relationship between RAS and survival.
Conclusions
Positive EGFR immunoexpression affects survival negatively, while RAS immunoexpression has no effect on survival in lung adenocarcinoma patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Hirsch FR, Scagliotti GV, Langer CJ, et al. Epidermal growth factor family of receptors in preneoplasia and lung cancer: perspectives for targeted therapies. Lung Cancer 2003;41 Suppl 1:S29-42. [PubMed]
- Nguyen DM, Schrump DS. Growth factor receptors as targets for lung cancer therapy. Semin Thorac Cardiovasc Surg 2004;16:3-12. [PubMed]
- Alberg AJ, Samet JM, et al. Epidemiology of lung cancer. Chest 2003;123:21S-49S. [PubMed]
- Jänne PA, Engelman JA, Johnson BE. Epidermal growth factor receptor mutations in non-small-cell lung cancer: implications for treatment and tumor biology. J Clin Oncol 2005;23:3227-34. [PubMed]
- Kozuki T, Hisamoto A, Tabata M, et al. Mutation of the epidermal growth factor receptor gene in the development of adenocarcinoma of the lung. Lung Cancer 2007;58:30-5. [PubMed]
- Liu Y, Xu ML, Zhong HH, et al. EGFR mutations are more frequent in well-differentiated than in poor-differentiated lung adenocarcinomas. Pathol Oncol Res 2008;14:373-9. [PubMed]
- Smith PW, Jones DR. Biology and epidemiology of lung cancer. In: Patterson GA, Cooper JD, Deslauriers J, et al. eds. Pearson’s Thoracic and Esophageal Surgery. 3rd Edition, Philadelphia, PA: Churchill Livingstone Elsevier, 2008:708-28.
- Meert AP, Martin B, Delmotte P, et al. The role of EGF-R expression on patient survival in lung cancer: a systematic review with meta-analysis. Eur Respir J 2002;20:975-81. [PubMed]
- Johnson BE, Heymach JV. Farnesyl transferase inhibitors for patients with lung cancer. Clin Cancer Res 2004;10:4254s-4257s. [PubMed]
- Osada H, Takahashi T. Genetic alterations of multiple tumor suppressors and oncogenes in the carcinogenesis and progression of lung cancer. Oncogene 2002;21:7421-34. [PubMed]
- Jasinski P, Zwolak P, Terai K, et al. Novel Ras pathway inhibitor induces apoptosis and growth inhibition of K-ras-mutated cancer cells in vitro and in vivo. Transl Res 2008;152:203-12. [PubMed]
- Zhu CQ, Shih W, Ling CH, et al. Immunohistochemical markers of prognosis in non-small cell lung cancer: a review and proposal for a multiphase approach to marker evaluation. J Clin Pathol 2006;59:790-800. [PubMed]
- Mascaux C, Iannino N, Martin B, et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer 2005;92:131-9. [PubMed]
- Strauss GM. Prognostic markers in resectable non-small cell lung cancer. Hematol Oncol Clin North Am 1997;11:409-34. [PubMed]
- Kanters SD, Lammers JW, Voest EE. Molecular and biological factors in the prognosis of non-small cell lung cancer. Eur Respir J 1995;8:1389-97. [PubMed]
- Sieber OM, Heinimann K, Tomlinson IP. Genomic instability--the engine of tumorigenesis? Nat Rev Cancer 2003;3:701-8. [PubMed]
- Sekido Y, Fong KM, Minna JD. Cancer of the Lung. In: DeVita VT Jr, Hellman S, Rosenberg SA. eds. Cancer, Principles and Practice of Oncology. 7th Edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2005:745-810.
- Holbro T, Civenni G, Hynes NE. The ErbB receptors and their role in cancer progression. Exp Cell Res 2003;284:99-110. [PubMed]
- Rusch V, Klimstra D, Venkatraman E, et al. Overexpression of the epidermal growth factor receptor and its ligand transforming growth factor alpha is frequent in resectable non-small cell lung cancer but does not predict tumor progression. Clin Cancer Res 1997;3:515-22. [PubMed]
- Volm M, Rittgen W, Drings P. Prognostic value of ERBB-1, VEGF, cyclin A, FOS, JUN and MYC in patients with squamous cell lung carcinomas. Br J Cancer 1998;77:663-9. [PubMed]
- Ohsaki Y, Tanno S, Fujita Y, et al. Epidermal growth factor receptor expression correlates with poor prognosis in non-small cell lung cancer patients with p53 overexpression. Oncol Rep 2000;7:603-7. [PubMed]
- Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer 2001;37 Suppl 4:S9-15. [PubMed]
- Niemiec J, Kolodziejski L, Dyczek S. EGFR LI and Ki-67 LI are independent prognostic parameters influencing survivals of surgically treated squamous cell lung cancer patients. Neoplasma 2005;52:231-7. [PubMed]
- Harada M, Dosaka-Akita H, Miyamoto H, et al. Prognostic significance of the expression of ras oncogene product in non-small cell lung cancer. Cancer 1992;69:72-7. [PubMed]
- Miyamoto H, Harada M, Isobe H, et al. Prognostic value of nuclear DNA content and expression of the ras oncogene product in lung cancer. Cancer Res 1991;51:6346-50. [PubMed]


