Impact of cannula design on packed red blood cell transfusions: technical advancement to improve outcomes in extracorporeal membrane oxygenation
Introduction
Over the last decade, extracorporeal membrane oxygenation (ECMO) has emerged as a promising intervention to provide supportive care to patients affected with acute respiratory failure (ARF) unresponsive to conventional and rescue therapies (1).
The survival rate of patients affected with ARF and supported with ECMO, in this case mainly in its veno-venous (VV) configuration, is progressively improving, but mortality is still high, even when bearing in mind that such support is usually initiated primarily in younger patients (2).
The improvement in clinical management of ECMO and its eventual complications on the one hand, and technological progress (3) on the other, have increased survival in specialized high-volume centers where a centralization process is established (4).
The rationale for ECMO use is to provide an adequate delivery of oxygen (DO2), which is assured by hemoglobin (Hb) content and blood flow in the circuit. For years now, Hb has been set at values close to the normal range (12–14 g/dL), but recent observational series (5,6) have suggested the feasibility of a lower transfusion threshold following the general reduction of transfusions in critically ill patients (7-9).
Apart from clinical considerations, the success of ECMO depends largely on the ability of the circuit to assure adequate blood flow, with oxygenation and decarboxylation over time (10). In the last few years, oxygenators and circuits have undergone an important improvement in efficacy and biocompatibility (11). The cannula (12) and the interface between patient and circuit have also changed, and cannula design has to be focused on maximizing the blood flow while causing minimal damage to the blood (13), the least possible coagulation activation (14), and easy placement. It is in this light that the HLS© BIOLINE-coated cannula have recently been introduced (15).
The aim of this study was to evaluate the impact of the introduction of new cannula specifically designed for ECMO on the number of packed red blood cells (pRBC) transfused in adult patients undergoing VV-ECMO, as well as its potential impact on reducing transfusion-related costs.
Methods
This study was approved by our Institutional Research Review Board and by the local Ethics Committee. All the patients in their full mental capacities, following recovery, signed an institutional consent for publication of manuscripts as anonymous aggregate data. The deceased patients were included, in accordance with authorization N° 9 of the Italian Privacy Law.
This is a single center retrospective study of consecutive adult patients supported on ECMO from February, 2008 to March, 2016. The patients were affected with acute respiratory distress syndrome (ARDS) as defined by the 1994 ARDS consensus or, later, the Berlin definition. Exclusion criteria for the study were <18 years of age, pre- or intra-operative support during lung transplantation or other surgical procedures (16), and veno-arterial configuration (combined respiratory/cardiac disease).
Medical history, demographics, biometrics, and lab tests were collected prospectively in our ECMO database through an electronic medical record system (Sunrise Clinical Manager, Allscripts Healthcare Solutions, Inc., Chicago, USA). ECMO predictive scores Predicting Death For Severe ARDS on VV-ECMO (PRESERVE), Respiratory Extracorporeal Membrane Oxygenation Survival Prediction score (RESP), and severity of illness scores Simplified Acute Physiology Score (SAPS-II) and Sepsis-related Organ Failure Assessment (SOFA) score were recorded at admission, as well. Acute kidney injury (AKI) was assessed according to the Acute Kidney Injury Network (AKIN) stage classification during the ECMO stay.
Transfusion requirement for pRBC was defined as the total number of units, total amount as mL, and median of mL transfused per day of ECMO support. Fresh frozen plasma (FFP) and platelets (PLTs) were counted as the number of patients who received transfusions and the volume of products transfused.
Bleeding episodes were classified as follows: overall (any kind of bleeding), minor (no intervention beyond stopping anticoagulation), major (>2 pRBC transfusions triggered by the bleeding event or interventional/surgical approach, including endoscopy, nasal/pharyngeal tamponade or cauterization), and fatal (intracerebral fatal hemorrhage) (17).
ECMO configuration and type of cannula
ECMO management was defined as type of device (ROTAFLOW or Cardiohelp System; Maquet; Getinge Group, Rastatt, Germany), cannulation configuration, and blood and sweep gas flow at the beginning and the last day of support. ECMO outcomes were defined as weaning from support, and effective intensive care unit (ICU) discharge.
All cannula was placed peripherally and percutaneously, and preferential ECMO configuration at our institute is with femoral drainage and right internal jugular reinfusion, while the femoro-femoral circuit is adopted when there are specific contraindications to jugular cannulation. Since the beginning of our ECMO program, we have placed our standard peripheral cannula for extracorporeal circulation (Edwards Lifesciences Corporation; Irvine, USA). The drainage cannula was thin-walled and wire-reinforced, with extended drainage holes: 20 and 22 Fr, 55 cm length, or 24 Fr and 68 cm for drainage. For reinfusion, we employed 16 Fr or, mainly, 18 Fr, 15 cm length for the jugular vein, and 20, 22 Fr, 55 cm length, or 24 Fr and 68 cm length for femoral reinfusion. In November, 2014 we introduced into our practice the HLS© cannula (Maquet; Getinge Group, Rastatt, Germany) with thin and biocompatible polyurethane bodies reinforced with a flat wire, alternating pairs of side holes, and coated with a BIOLINE matrix composed of albumin and heparin for an increased tip-to-tip biocompatible circuit certified for an extended use of up to 30 days: 23 and 25 Fr, 38 cm, and 17 or 19 Fr, 15 cm length for jugular vein reinfusion (in one case 23 Fr, 55 cm as femoral reinfusion).
Institutional protocol for anticoagulation and transfusions during ECMO
Our protocol for transfusions and anticoagulation (5) is based on integration of the Hb values with other parameters: mixed venous oxygen saturation (SvO2), urine output, lactates, and hemodynamics. Hematocrit (Htc) is maintained between 24% and 30% unless severe hypoxia is present despite rescue maneuvers. Anticoagulation is maintained with heparin at a target range of activated partial thromboplastin time (APTT) between 40 and 50 seconds, checked every four hours, and stopped in any case of bleeding or in case of spontaneous coagulopathy. Antithrombin III (AT III) activity is checked daily and replaced by AT III concentrates, as needed, to keep the activity as near as possible at 100%. Blood waste is avoided: need for blood sampling is re-evaluated daily, and at ECMO weaning the blood in the circuit is reinfused in the patient.
PLTs are transfused when the level falls below 40,000–50,000/mL, according to the identification of possible bleeding sources. FFP is transfused when there is evidence of relevant bleeding in the presence of coagulopathy (18).
Statistical analyses
Categorical variables are given as frequencies and percentages, continuous variables as mean and standard deviation or as median and interquartile range (IQR) (25th–75th percentile IQR), when appropriate, according to data distribution.
The comparison between the BIOLINE group and the Standard cannula group was made with the two-sample t-test for continuous variables or Wilcoxon rank-sum test, and by Fisher’s exact test or Chi-square test for categorical variables, when appropriate.
All tests were two-sided, and a p value of <0.05 was considered indicative of statistical significance. Data handling and analyses were done with SAS 9.4 software (SAS Institute Inc., Cary, NC, USA).
Results
During the study period, 92 patients required ECMO support for ARF at our institute. Eighty-nine patients comprised our study cohort since 3 were excluded for the following reasons: 2 affected with hematologic malignancies (inappropriate to explore the topic of transfusion), 1 because at too high a risk of death (SAPS-II at ICU admission 66 and SOFA score 18) and with less than 48 hours of ICU stay.
The patients were divided into two groups according to the type of cannulation: group 1, named Standard, was composed of 52 patients with Edwards cannula, and group 2, named BIOLINE, and composed of 37 patients with HLS© BIOLINE cannula.
The underlying diagnoses were H1N1 influenza A (27 patients in the Standard group and 17 patients in the BIOLINE group), bacterial pneumonia (12 Standard and 16 BIOLINE), lung graft failure (6 cases in the Standard group), polytrauma (2 Standard, 1 BIOLINE), pneumocystis jirovecii pneumonia in HIV-AIDS (1 Standard, 2 BIOLINE), ARF post-pneumonectomy (2 cases in the Standard group), mediastinitis (1 case in the Standard group), chemical pneumonia (1 case in the Standard group), and 1 case of transfusion-related lung injury in the BIOLINE group.
Patient characteristics of the combined cohort and separate groups are summarized in Table 1. The two groups were comparable in terms of the majority of baseline and pre-ECMO characteristics.
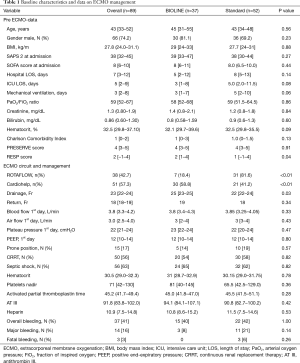
Full table
ECMO management
No relevant between-group differences were found in ECMO management apart from the type of console, and diameter and length of drainage cannula. All parameters for the targets for transfusions and anticoagulation (Htc, platelet nadir, APTT, AT III, heparin) were comparable between the two groups, and in range for our protocol (all P values were >0.05) (Table 1). In the BIOLINE group there was a higher prevalence of Cardiohelp adoption, while in the Standard group there was more frequent use of the ROTAFLOW console and pump (P<0.001). In the BIOLINE group there was a higher median diameter of the inflow cannula: 25 Fr, IQR 23–25 vs. 22 Fr, IQR 22–24, P=0.03.
Transfusion management and bleeding
The overall cohort received a reduced number and amount of pRBC transfusions related to our adaptable and restrictive protocol: the median number of pRBC units transfused was 8 (IQR 3–18), and the amount in ml adjusted for the duration of ECMO was 158 mL pRBC/day of ECMO support (IQR 91–214), with 9 patients who did not require any pRBC units (Table 2).

Full table
Investigating different management in the two groups we found significantly fewer pRBC transfusions in the group with BIOLINE cannulation (Table 2). This is evident considering the total number of transfused units during the ECMO stay: 4 (IQR 1–9) in the BIOLINE group, 12 (IQR 5.5–21) in the Standard group (P=0.004). The evidence is even more relevant considering the daily amount: 91 mL/day ECMO (IQR 21–158) in the BIOLINE and 193.5 mL/day ECMO (IQR 140.5–254) in the Standard group (P<0.001). Moreover, in the BIOLINE group, 9 patients received no pRBC transfusion during the ECMO duration (mean ECMO length 9±6 days).
FFP was transfused in 11 of the 89 patients, and in a comparable way between the two groups: 5 patients in the BIOLINE group, and 6 patients in the Standard group. PLTs were transfused in 28 of the 89 patients: 9 in the BIOLINE group, and 19 in the Standard group.
There was no statistically significant difference between the groups in terms of bleeding episodes (Table 1). Apart from 3 fatal bleedings, i.e., intracranial hemorrhage, we registered overall bleeding in 15 cases in the BIOLINE group, and 22 in the Standard group. Episodes of major bleeding were less frequent with the adoption of the new cannula, though not statistically significant (P=0.140). In the BIOLINE group we registered 3 major bleedings: 1 due to duodenal gastric ulcer, 1 due to rhinopharyngeal and abdominal bleeding in a polytrauma patient, 1 due to cannulation-site-relevant bleeding. In the standard group we encountered 11 major bleedings: 4 were intracerebral hemorrhage (in 3 cases they were fatal, while in 1 case a surgical evacuation of hematoma allowed recovery); 3 were hemothorax (2 patients on ECMO for lung graft failure, and 1 polytrauma patient who underwent a thoracotomy during ECMO support, all 3 successfully weaned); 2 were severe gastric bleeding (one recognized as an incidental diagnosis of a gastric bleeding cancer); 1 case was a rhinopharyngeal and gastric bleeding with several interventional procedures to stop the bleeding in a patient with a long ECMO stay and an acquired von Willebrand disease; and 1 case of cannulation bleeding in a patient with heparin-induced thrombocytopenia.
Clinical outcomes
The number of days on ECMO evidenced a borderline statistical significance, with a reduction using the new and more biocompatible devices (P=0.05) (Table 3) This reduction in ECMO duration is also reflected in the trend of reduction in total ICU length of stay (LOS), while there was no difference in the median number of days of mechanical ventilation post-ECMO. ECMO survival (namely the successful weaning of the extracorporeal device) and effective discharge from the ICU were comparable in the two groups.
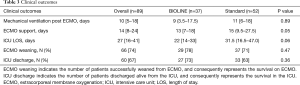
Full table
Costs of transfusions and cannula
Considering that at our institute every unit of pRBC has a total cost of €224.40, the median cost for pRBC during ECMO in the BIOLINE group was €897.60 per treatment per patient, while in the Standard group the median cost was €2,692.80. This gain in cost effectiveness was partially offset by the higher cost of the cannula: we calculated an increased cost for cannulation of €500.00 in the BIOLINE group. Consequently, the implementation of the new BIOLINE cannulation resulted in a median saving of about €1,295.20 considering just pRBC and cannula, and not including the potential reduction in ECMO duration and ICU LOS.
Discussion
In this study, we evaluated different transfusion results in VV-ECMO patients treated with two different types of cannula.
Currently, several approaches are adopted to improve the biocompatibility of extracorporeal circuits (19-21), based on the use of antithrombotic biomolecules such as heparin (22), polymeric molecules, and glycoprotein because the ECMO circuit is one of the largest and longest surface areas and volume for blood contact (23) in any medical device for prolonged use (10,24).
The BIOLINE coating combines albumin and heparin polypeptide absorbed into the components of the circuits’ surfaces, forming a steric hindrance. With heparin, a negatively charged hydrophilic complex polysaccharide acid, molecules are attached to the polypeptides via covalent bonds and ionic interaction. It has been hypothesized that the circuits’ higher biocompatibility, combined with a low median heparin dose, restricted range of APTT, and a high median AT III (25), may cause less derangement of the physiological coagulation/anticoagulation balance (26).
In terms of ECMO management, we found a difference in pump and console use because the more recent cases were treated mainly with the Cardiohelp system (27). Therefore, we progressively switched from ROTAFLOW use to Cardiohelp (both polymethylpentene membrane oxygenators) because almost all our patients are referred by other hospitals (on-site cannulated) and, consequently, we used the properly miniaturized new console (28) for transport (29). This difference may have influenced the results since the Cardiohelp provides a slightly lower priming volume, and the difference in the circuit may reduce the formation of vortices, thus reducing the potential for hemolysis. The dynamic differences between the two systems have been not completely elucidated by the literature, but there are some data suggesting a reduced pressure drop with the Cardiohelp system, and the ability to achieve desired flow rates at lower rotational speeds (30). Moreover, the Cardiohelp offers the opportunity of close monitoring of the circuit’s pressures and Hb values, which can help for a more tailored fluid and blood administration. This is the main bias of our data collection since these two systems are quite different in terms of biodynamics and monitoring; but at the same time, it is an evidence of updated clinical practice, which is also the basis of research in biomedical technology.
The second difference between groups concerns the drainage cannula. In the current literature there is consensus concerning the best configuration (2)—femoro-jugular, based on expert advice, though there is no ultimate definition of the best cannula type, size, and length (31,32). In the evolution of our cannulation approach a relevant role has been played by the different designs (33), accompanied by a shorter length of the drainage cannula, an active way to reduce circuit length. In fact, adequate management of the patient’s intravascular volume status is critical during ECMO; therefore, we can speculate that a lower speed of the pump with a stable blood flow may translate into a lower number of pRBC transfusions, and better oxygenation may reduce the peripheral tissue hypoxia and organ failure, favoring the patient’s recovery (34).
These last considerations involve the strategy of managing the ARF patient on ECMO support, the rationale of which is to provide variable but partial oxygenation of the entire cardiac output. Before administering a transfusion (35) to increase the DO2 it is considered important to check for blood flow, optimize ventilation when possible, and reduce oxygen expenditure, all of which constitute a plausible pathway toward the application of the principles of precision medicine for all ECMO patients.
Considering the bleeding complications, we found a reduced number of overall episodes in the BIOLINE group, but without statistical significance. Theoretically, a less relevant derangement of physiologic coagulation may reduce the episodes of bleeding due to coagulopathy, but several considerations may put into question definitive conclusions from our series since the differences among the episodes of bleeding in the two groups were more related to the case mix and the reduced number of ECMO runs, though we cannot exclude a potential role in the reduced number of days of ECMO support.
Finally, we looked at costs of transfusions. This is a non-negligible “side-effect” of transfusions considering the costs incurred for the health care system, and the shortage of pRBC in blood banks. The use of BIOLINE cannula is much more expensive than the older cannula, but considering the potential for reduction by one-third of pRBC administration, expenses can be considered cost-effective (36). This hypothesis is even more relevant in light of potentially reduced length of ECMO support, and should receive increasing interest in the ECMO community since the applicability of ECMO (37,38) is constantly spreading, even toward pathologies not traditionally associated with ECMO support (39-42). Obviously, this is not a cost-analysis since even the change in the ECMO system is expensive and increases the costs, though it is an example of the indirect clinical costs and savings that can be derived from a careful consideration of the available techniques.
Our study has several limitations. First, it is a single center study and with a retrospective design. Second, apart from a few cases, the two groups were not treated contemporaneously, since the standard cannula were adopted mainly in a previous period; consequently, our data are probably influenced by the learning curve and the general improvement in ICU knowledge. In addition, a confounding factor may be the later use of new devices, which have an integrated monitoring of Hb and SvO2.
Despite such limitations, we believe that our study has some strengths. To the best of our knowledge, this is the first study to compare different types of cannula in transfusions. This is a large and homogeneous cohort of adult VV-ECMO patients affected with ARF, and despite the almost before-after design of the study the principles of management did not change over the years, as evidenced by the similar levels of Htc, APTT, and heparin. In addition, our data collection methods were consistent and computer-based, also adding the costs of transfusions and ECMO technology, thus offering a practical and “real life” picture of the field. Lastly, our conclusions are hypothesis-generating more than evidence statements and highlight the importance of careful consideration of circuit configurations and type of cannula use.
Conclusions
Our results suggest that the use of shorter and more-biocompatible cannula, associated with a restrictive policy of anticoagulation and transfusion, may contribute to a reduction in transfusions during VV-ECMO and to a potentially indirect more cost-effective practice. Our results should be considered hypothesis-generating and highlight the importance of further investigation in prospective studies of the specific effects of the clinical advancements determined by new and evolving technologies during ECMO.
Acknowledgements
We are indebted to Warren Blumberg, science editor at ISMETT, for his help in revising the manuscript, to Dr. Diego Bellavia, who performed the first interim analysis, to Drs. Cristina Santonocito and Filippo Sanfilippo, who contributed to acquiring the informed consent from the recovered patients, and to Gaspare Di Lorenzo Chief of Perfusion Service who contributed to data collection. Partial and preliminary data were presented at the 70th SIAARTI national congress in Naples, 26/10–29/10, 2016.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by ISMETT’s Institutional Research Review Board and by the local Ethics Committee (Comitato Etico Palermo 2; Reference Code: 88-ISMETT-2016).
References
- Bein T, Grasso S, Moerer O, et al. The standard of care of patients with ARDS: ventilatory settings and rescue therapies for refractory hypoxemia. Intensive Care Med 2016;42:699-711. [Crossref] [PubMed]
- Fan E, Gattinoni L, Combes A, et al. Venovenous extracorporeal membrane oxygenation for acute respiratory failure: A clinical review from an international group of experts. Intensive Care Med 2016;42:712-24. [Crossref] [PubMed]
- Biscotti M, Lee A, Basner RC, et al. Hybrid configurations via percutaneous access for extracorporeal membrane oxygenation: a single-center experience. ASAIO J 2014;60:635-42. [Crossref] [PubMed]
- Combes A, Brodie D, Bartlett R, et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med 2014;190:488-96. [Crossref] [PubMed]
- Martucci G, Panarello G, Occhipinti G, et al. Anticoagulation and Transfusions Management in Veno-Venous Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome: Assessment of Factors Associated With Transfusion Requirements and Mortality. J Intensive Care Med 2017. [PubMed]
- Mazzeffi M, Greenwood J, Tanaka K, et al. Bleeding, Transfusion, and Mortality on Extracorporeal Life Support: ECLS Working Group on Thrombosis and Hemostasis. Ann Thorac Surg 2016;101:682-9. [Crossref] [PubMed]
- Lelubre C, Vincent JL, Taccone FS. Red blood cell transfusion strategies in critically ill patients: lessons from recent randomized clinical studies. Minerva Anestesiol 2016;82:1010-6. [PubMed]
- Esper SA, Welsby IJ, Subramaniam K, et al. Adult extracorporeal membrane oxygenation: an international survey of transfusion and anticoagulation techniques. Vox Sang 2017;112:443-52. [Crossref] [PubMed]
- Smith A, Hardison D, Bridges B, et al. Red blood cell transfusion volume and mortality among patients receiving extracorporeal membrane oxygenation. Perfusion 2013;28:54-60. [Crossref] [PubMed]
- Kohler K, Valchanov K, Nias G, et al. ECMO cannula review. Perfusion 2013;28:114-24. [Crossref] [PubMed]
- Tay CK, Sung K, Cho YH. Clinical Pearls in Venovenous Extracorporeal Life Support for Adult Respiratory Failure. ASAIO J 2018;64:1-9. [Crossref] [PubMed]
- Reeb J, Olland A, Renaud S, et al. Vascular access for extracorporeal life support: tips and tricks. J Thorac Dis 2016;8:S353-63. [Crossref] [PubMed]
- Toomasian JM, Bartlett RH. Hemolysis and ECMO pumps in the 21st Century. Perfusion 2011;26:5-6. [Crossref] [PubMed]
- Bolliger D, Zenklusen U, Tanaka KA. Point-of-care coagulation management algorithms during ECMO support: are we there yet? Minerva Anestesiol 2016;82:1000-9. [PubMed]
- Palatianos GM, Foroulis CN, Vassili MI, et al. A prospective, double-blind study on the efficacy of the bioline surface-heparinized extracorporeal perfusion circuit. Ann Thorac Surg 2003;76:129-35. [Crossref] [PubMed]
- Buscher H, Vukomanovic A, Benzimra M, et al. Blood and Anticoagulation Management in Extracorporeal Membrane Oxygenation for Surgical and Nonsurgical Patients: A Single-Center Retrospective Review. J Cardiothorac Vasc Anesth 2017;31:869-75. [Crossref] [PubMed]
- Martucci G, Lo Re V, Arcadipane A. Neurological injuries and extracorporeal membrane oxygenation: the challenge of the new ECMO era. Neurol Sci 2016;37:1133-6. [Crossref] [PubMed]
- Bolliger D, Tanaka KA. Point-of-care coagulation tests and medication-induced hypertriglyceridemia in extracorporeal membrane oxygenation patients. Minerva Anestesiol 2017;83:891. [PubMed]
- Zimmermann AK, Weber N, Aebert H, et al. Effect of biopassive and bioactive surface-coatings on the hemocompatibility of membrane oxygenators. J Biomed Mater Res B Appl Biomater 2007;80:433-9. [Crossref] [PubMed]
- Pappalardo F, Montisci A, Scandroglio A, et al. Veno-Venous ECMO in Europe: are we all speaking the same language? Minerva Anestesiol 2017;83:424-5. [PubMed]
- Reser D, Seifert B, Klein M, et al. Retrospective analysis of outcome data with regards to the use of Phisio(R)-, Bioline(R)- or Softline(R)-coated cardiopulmonary bypass circuits in cardiac surgery. Perfusion 2012;27:530-4. [Crossref] [PubMed]
- Hsu LC. Heparin-coated cardiopulmonary bypass circuits: current status. Perfusion 2001;16:417-28. [Crossref] [PubMed]
- Hirsh SL, McKenzie DR, Nosworthy NJ, et al. The Vroman effect: competitive protein exchange with dynamic multilayer protein aggregates. Colloids Surf B Biointerfaces 2013;103:395-404. [Crossref] [PubMed]
- Tulman DB, Stawicki SP, Whitson BA, et al. Veno-venous ECMO: a synopsis of nine key potential challenges, considerations, and controversies. BMC Anesthesiol 2014;14:65. [Crossref] [PubMed]
- Ranucci M, Cazzaniga A, Soro G, et al. The antithrombin III-saving effect of reduced systemic heparinization and heparin-coated circuits. J Cardiothorac Vasc Anesth 2002;16:316-20. [Crossref] [PubMed]
- Martens S, Matheis G, Wimmer-Greinecker G, et al. Heparin coating of the extracorporeal circuit combined with leukocyte filtration reduces coagulation activity, blood loss and blood product substitution. Int J Artif Organs 2001;24:484-8. [Crossref] [PubMed]
- Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 2011;365:1905-14. [Crossref] [PubMed]
- Philipp A, Arlt M, Amann M, et al. First experience with the ultra compact mobile extracorporeal membrane oxygenation system Cardiohelp in interhospital transport. Interact Cardiovasc Thorac Surg 2011;12:978-81. [Crossref] [PubMed]
- Haneya A, Philipp A, Foltan M, et al. First experience with the new portable extracorporeal membrane oxygenation system Cardiohelp for severe respiratory failure in adults. Perfusion 2012;27:150-5. [Crossref] [PubMed]
- Undar A, Wang S, Moroi M, et al. Evaluation and Comparison of Hemodynamic Performance of Three ECLS Systems in a Simulated Adult Cardiogenic Shock Model. Artif Organs 2018;42:776-85. [Crossref] [PubMed]
- Palmer O, Palmer K, Hultman J, et al. Cannula Design and Recirculation During Venovenous Extracorporeal Membrane Oxygenation. ASAIO J 2016;62:737-42. [Crossref] [PubMed]
- Burns J, Cooper E, Salt G, et al. Retrospective Observational Review of Percutaneous Cannulation for Extracorporeal Membrane Oxygenation. ASAIO J 2016;62:325-8. [Crossref] [PubMed]
- Park JY, Park CY, Min BG. A numerical study on the effect of side hole number and arrangement in venous cannulae. J Biomech 2007;40:1153-7. [Crossref] [PubMed]
- Spinelli E, Bartlett RH. Relationship between hemoglobin concentration and extracorporeal blood flow as determinants of oxygen delivery during venovenous extracorporeal membrane oxygenation: a mathematical model. ASAIO J 2014;60:688-93. [Crossref] [PubMed]
- Tauber H, Streif W, Fritz J, et al. Predicting Transfusion Requirements During Extracorporeal Membrane Oxygenation. J Cardiothorac Vasc Anesth 2016;30:692-701. [Crossref] [PubMed]
- Ejaz A, Frank SM, Spolverato G, et al. Potential Economic Impact of Using a Restrictive Transfusion Trigger Among Patients Undergoing Major Abdominal Surgery. JAMA Surg 2015;150:625-30. [Crossref] [PubMed]
- Roncon-Albuquerque R Jr, Almeida V, Lopes M, et al. Cost analysis of miniaturized ECMO in H1N1-related ARDS managed by a single caregiver. Intensive Care Med 2014;40:910-1. [Crossref] [PubMed]
- Cavarocchi NC, Wallace S, Hong EY, et al. A cost-reducing extracorporeal membrane oxygenation (ECMO) program model: a single institution experience. Perfusion 2015;30:148-53. [Crossref] [PubMed]
- Martucci G, Panarello G, Bertani A, et al. Veno-venous ECMO in ARDS after post-traumatic pneumonectomy. Intensive Care Med 2013;39:2235-6. [Crossref] [PubMed]
- Di Lorenzo G, Martucci G, Sciacca S, et al. Dysfunction of mechanical mitral prosthesis at 33rd week of pregnancy: ECMO support as a complex strategy for the mother and the fetus. Perfusion 2016;31:611-3. [Crossref] [PubMed]
- Martucci G, Burgio G, Lullo F, et al. Veno-arterial extracorporeal membrane oxygenation as an intraoperative rescue option in case of portopulmonary hypertension recognized during liver transplantation. Minerva Anestesiol 2017;83:1336-7. [PubMed]
- Panholzer B, Meckelburg K, Huenges K, et al. Extracorporeal membrane oxygenation for acute respiratory distress syndrome in adults: an analysis of differences between survivors and non-survivors. Perfusion 2017;32:495-500. [Crossref] [PubMed]


