
The importance of PD-L1 diagnostic assay harmonization for the selection of lung cancer immunotherapy
The introduction of immune checkpoint inhibitors (ICIs), antibodies that target co-inhibitory molecules to improve anti-tumor immune responses, has dramatically changed the therapeutic landscape of several malignancies, particularly that of lung cancer (1,2).
Tumor biomarkers are associated with specific molecular pathway alterations that, in some cases, may be biologically necessary or sufficient to drive cancer pathogenesis, in which case they represent potentially actionable molecular targets for the development of targeted drugs. It is now very common for early-phase clinical trials to use biomarkers to enrich trial populations with patients that are more likely to benefit from the drug being tested. This strategy has enabled to simultaneously test an experimental agent together with the diagnostic assay developed alongside.
Although the drug-diagnostic co-development model has accelerated the rate at which targeted drugs receive clinical approval, it has also coupled specific therapeutic agents with their own biomarker assay, as it is the case for tyrosine kinase inhibitors and the presence of EGFR activating mutations or ALK and ROS1 translocations. However, in the case of anti-PD-1/PD-L1 therapies, there are up to five different drugs, each with its own, independent and trial-validated immunohistochemistry (IHC)-based biomarker assay. Oftentimes, biopsy tissue is not sufficient to perform multiple IHC-based assays and genomic-based assays. Furthermore, testing for numerous biomarkers dramatically increases costs, which makes patients and publicly funded healthcare institutions less able to absorb these expenses. Therefore, unlike other tumor-biomarker tests that are routinely performed by pathologists, PD-L1 testing is requested by the oncologist who decides which assay should be performed, based on the drug which he/she intends to use. This has posed a new set of challenges for pathologists and oncologists, all of which have been described extensively elsewhere (3-5).
PD-1, programmed cell death 1 protein, acts as an inhibitory molecule on the surface of immune cells, normally working to prevent tissue damage arising from excessive inflammation. However, in the tumor microenvironment, binding of PD-1 with its ligands (PD-L1 and PD-L2) protects tumor cells from cytotoxic T-cell attack, thus facilitating tumor immune evasion. The development of ICIs to restore antitumor immunity has therefore opened a new frontier in cancer therapeutics (1-3). It is for this reason that we read with great interest the review of Ancevski Hunter et al. (“PD-L1 Testing in Guiding Patient Selection for PD-1/PD-L1 Inhibitor Therapy in Lung Cancer”) (6). The authors provided a comprehensive review about the pivotal trials that led to the approval of anti-PD-1/PD-L1 ICIs for the treatment of non-small cell lung cancer (NSCLC) while highlighting the role of specific diagnostic assays during the approval of each of the agents discussed. It is unfortunate that publication occurred prior to the 2018 Annual Meeting of both the American Society of Clinical Oncology (ASCO 2018) and the American Association for Cancer Research (AACR 2018), where relevant and exciting new results were presented, their insight would have been much welcomed.
We do feel that it is pertinent to further emphasize the difference between “complementary” and “companion” diagnostics as it pertains to the regulatory approval and indication of nivolumab, atezolizumab and pembrolizumab. The US Food and Drug Administration (FDA) defines a “companion diagnostic” as “a medical device, often an in vitro device, which provides information that is essential for the safe and effective use of a specific drug or biological product” within its approved labeling. The first assay to obtain this regulatory approval was HercepTest® (DAKO, Agilent Technologies Company), a semi-quantitative IHC assay to determine HER2 protein overexpression, which is linked to the use of Trastuzumab (Herceptin®), a humanized anti-HER2 monoclonal antibody (mAb) (7). It was estimated that in 2017 the FDA had approved approximately 20 anticancer drugs, each linked to a companion diagnostic test (8).
In contrast, a “complementary diagnostic” assay is a test that aids in the therapeutic decision process but that is not required when prescribing the corresponding drug, since it is not harmful to treat patients with the associated drug in the absence of assay results or if the results are negative (9). However, it is important to clarify that performing a complementary diagnostic assay is highly recommended. In 2015 the PD-L1 IHC 28-8 PharmDx assay (DAKO, Glostrop, Denmark) became the first assay to obtain regulatory approval as a “complementary diagnostic” when the FDA simultaneously approved nivolumab (OPDIVO; Bristol-Myers Squibb, New York, NY) for second-line treatment of non-squamous NSCLC. This new regulatory approval may reflect the notion that patients should not be excluded from receiving cancer immunotherapies when there is not enough evidence showing that treatment efficacy is strongly dependent on higher levels of tumor PD-L1 expression (7).
For instance, results from CheckMate-017 (10) and CheckMate-063 (11) showed that tumor PD-L1 expression was neither prognostic nor predictive of benefit to second-line nivolumab monotherapy in non-squamous NSCLC. On the other hand, results from CheckMate-057 showed that tumor PD-L1 expression was predictive of benefit to second-line nivolumab therapy (12) in non-squamous NSCLC but increasing PD-L1 tumor proportion score (TPS; 1%, 5% and 10%) only resulted in a moderate increase in the response rate of patients (12). Results from Checkmate 012 indicated that first-line nivolumab monotherapy elicited durable responses in patients with advanced NSCLC, regardless of tumor PD-L1 expression (13). These results were inconsistent with those from CheckMate 026, where first-line nivolumab monotherapy was not associated with significantly longer progression-free survival (PFS), or overall survival (OS), compared to chemotherapy [4.2 vs. 5.9 months; hazard ratio (HR) =1.15; 95% CI, 0.91–1.45; P=0.25] in patients with NSCLC and tumor PD-L1 expression ≥5% (14,15). Furthermore, the lack of benefit persisted even among patients with PD-L1 expression ≥50% (HR =1.07; 95% CI, 0.77–1.49).
In contrast, the results from KEYNOTE-024 showed that first-line pembrolizumab monotherapy was associated with improved PFS (PFS 10.3 months with pembrolizumab 6.0 months with chemotherapy; HR =0.50; 95% CI, 0.37–0.68; P<0.001) in NSCLC patients with PD-L1 expression ≥50% (16).
Although it is not valid to compare results from trials with different experimental designs, it is becoming increasingly difficult to ignore the discrepancies between Checkmate 012 (13) and CheckMate-026 (14,15), as well as the conflicting results between CheckMate-026 and KEYNOTE-024 (16), particularly in light of the preliminary results from KEYNOTE-042 presented in ASCO 2018, showing that first-line pembrolizumab monotherapy significantly improved OS, as compared to platinum-based chemotherapy (16.7 vs. 12.2 months; HR =0.81; 95% CI, 0.71–0.93; P=0.0018), in patients with advanced NSCLC and PD-L1 TPS ≥1%. Responses were more durable with pembrolizumab than with chemotherapy at all levels of PD-L1 expression, but clinical benefit increased with higher levels of PD-L1 expression (17), which is consistent with previous results and supports the use of pembrolizumab as first-line monotherapy in patients with PD-L1 expression greater than 50%. It remains to be seen if these results will lead to an expanded approval for pembrolizumab by the FDA and whether the PD-L1 IHC 22C3 pharmDx assay will continue to be a companion diagnostic (with adjusted cut-off values), a point that will be expanded later on.
It has generally been accepted that the pharmacologic and biologic properties of these two mAbs do not differ significantly, making them virtually interchangeable (18), and that discrepancies between these two trials are primarily due to differences in patient characteristics as well as due to assays variations and cut-off points used to evaluate PD-L1 expression and to select eligible patients (Table 1) (19). However, to the best of our knowledge, there are no clinical studies with a design that would allow for a head-to-head comparison between the efficacy of different anti-PD-1/PD-L1 therapeutic mAbs and their corresponding diagnostic assays. We believe that it is essential that subsequent studies, both prospective and retrospective, evaluate in more detail the clinicopathological characteristics that have been suggested to affect the response and survival of NSCLC patients. If indeed it is verified that different patient characteristics (across trials) and imbalanced experimental and active comparator arms (within trials) affected drug efficacy, then these factors could and should be used to identify patients that are more likely to benefit from therapy with either agent.
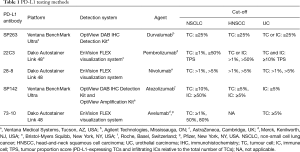
Full table
Instead, great efforts and resources have been allocated to achieve “assay harmonization” in order to determine to what extent treatment responses and survival outcomes would be reproducible if different assays were used to select patients for a specific treatment. Consequently, there have been a number of comparative studies assessing the technical performance assays using the same set of NSCLC tumor samples, showing that the 28-8, 22C3, and E1L3N assay, but not the SP142 assay, yield similar tumor staining results (20), which is not surprising considering that the SP142 clone was optimized to stain immune infiltrating cells. Regarding the staining variability of archival samples of different ages, there is evidence indicating that there are no significant differences in the prevalence of PD-L1 between blocks that are less than 3 years old (21). Scheel et al. recently published a study showing that inaccuracy of scoring due to interobserver discordance is less than 10% (22). Similarly, it has been reported that if different assays and cutoff points were used to assess PD-L1 expression, this would lead to a change in the treatment allocation of 10–15% patients (23). Ratcliffe et al. (24) presented a comparative study of three commercially available, trial-validated assays based on 28-8, 22C3, and SP263 antibodies. This study showed that the technical performance of these three assays was very similar, with greater than 90% overall agreement in all comparisons across the total range of PD-L1 expression. In the same vein, Adam et al. showed a high concordance for tumor cells staining across the five Dako, Ventana and Leica platforms. Additionally, the clone SP263 achieved the highest concordance rate across all platforms (25).
In the absence of comparable clinical data regarding the efficacy of similar therapeutic agents, we concur with the authors that standardizing the different diagnostic assays, and their scoring, is an important first step towards providing patients with consistent information regarding the probability of achieving a beneficial therapeutic outcome with a specific treatment. Another topic to keep in mind is how the emergence of other predictive biomarkers (such as high tumor mutation burden, cancer-associated microRNA expression, neo-antigen expression and the diversity of tumor antigen-specific T cells) will impact the utility of assessing PD-L1 expression alone. It is important to evaluate whether the simultaneous assessment of several markers could be used to better outline patient selection. As an example, perhaps PD-L1 expression could be assessed in conjunction with the expression of lymphocyte markers or MHC-II molecules to more accurately predict the therapeutic benefit that a patient may derive from anti-PD-1. Lastly, it is essential that the consistency between biomarkers across neoplasms be contemplated throughout this validation process.
Acknowledgements
None.
Footnote
Conflicts of Interest: AF Cardona discloses financial research support from Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol-Myers Squibb and The Foundation for Clinical and Applied Cancer Research (FICMAC). Additionally, he was linked to and received honoraria as advisor, participated in speakers’ bureau and gave expert testimony to Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Pfizer, Novartis, Celldex Therapeutics, Foundation Medicine, Eli Lilly and Foundation for Clinical and Applied Cancer Research (FICMAC). Oscar Arrieta has received honoraria as advisor, participated in speakers’ bureau and gave expert testimony to Pfizer, AstraZeneca, Boehringer-Ingelheim, Roche, Lilly and Bristol-Myers Squibb. The other authors have no conflicts of interest to declare.
References
- Rizvi H, Sanchez-Vega F, La K, et al. Molecular Determinants of Response to Anti-Programmed Cell Death (PD)-1 and Anti-Programmed Death-Ligand 1 (PD-L1) Blockade in Patients With Non-Small-Cell Lung Cancer Profiled With Targeted Next-Generation Sequencing. J Clin Oncol 2018;36:633-41. [Crossref] [PubMed]
- Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol 2018;29:84-91. [Crossref] [PubMed]
- Kerr KM, Hirsch FR. Programmed Death Ligand-1 Immunohistochemistry: Friend or Foe? Arch Pathol Lab Med 2016;140:326-31. [Crossref] [PubMed]
- Kerr KM, Nicolson MC. Non-Small Cell Lung Cancer, PD-L1, and the Pathologist. Arch Pathol Lab Med 2016;140:249-54. [Crossref] [PubMed]
- Kerr KM, Tsao MS, Nicholson AG, et al. Programmed Death-Ligand 1 Immunohistochemistry in Lung Cancer: In what state is this art? J Thorac Oncol 2015;10:985-9. [Crossref] [PubMed]
- Ancevski Hunter K, Socinski MA, Villaruz LC. PD-L1 Testing in Guiding Patient Selection for PD-1/PD-L1 Inhibitor Therapy in Lung Cancer. Mol Diagn Ther 2018;22:1-10. [Crossref] [PubMed]
- Scheerens H, Malong A, Bassett K, et al. Current Status of Companion and Complementary Diagnostics: Strategic Considerations for Development and Launch. Clin Transl Sci 2017;10:84-92. [Crossref] [PubMed]
- Hersom M, Jorgensen JT. Companion and Complementary Diagnostics-Focus on PD-L1 Expression Assays for PD-1/PD-L1 Checkpoint Inhibitors in Non-Small Cell Lung Cancer. Ther Drug Monit 2018;40:9-16. [PubMed]
- Milne CP, Bryan C, Garafalo S, et al. Complementary versus companion diagnostics: apples and oranges? Biomark Med 2015;9:25-34. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2980-7. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Socinski M, Creelan B, Horn L, et al. CheckMate 026: A phase 3 trial of nivolumab vs investigator's choice of platinum-based doublet chemotherapy as first-line therapy for stage IV/recurrent programmed death ligand 1-positive NSCLC. Ann Oncol 2016;27:LBA7_PR. Available online: https://doi.org/ [Crossref]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Lopes G, Wu YL, Kudaba L, et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥1%: Open-label, phase 3 KEYNOTE-042 study. J Clin Oncol 2018;36: abstr LBA4.
- Prasad V, Kaestner V. Nivolumab and pembrolizumab: Monoclonal antibodies against programmed cell death-1 (PD-1) that are interchangeable. Semin Oncol 2017;44:132-5. [Crossref] [PubMed]
- Remon J, Besse B, Soria JC. Successes and failures: what did we learn from recent first-line treatment immunotherapy trials in non-small cell lung cancer? BMC Med 2017;15:55. [Crossref] [PubMed]
- Rimm DL, Han G, Taube JM, et al. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol 2017;3:1051-8. [Crossref] [PubMed]
- Midha A, Sharpe A, Scott M, et al. PD-L1 expression in advanced NSCLC: Primary lesions versus metastatic sites and impact of sample age. J Clin Oncol 2016;34:abstr 3025.
- Scheel AH, Dietel M, Heukamp LC, et al. Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. Mod Pathol 2016;29:1165-72. [Crossref] [PubMed]
- Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol 2017;12:208-22. [Crossref] [PubMed]
- Ratcliffe MJ, Sharpe A, Midha A, et al. Agreement between Programmed Cell Death Ligand-1 Diagnostic Assays across Multiple Protein Expression Cutoffs in Non-Small Cell Lung Cancer. Clin Cancer Res 2017;23:3585-91. [Crossref] [PubMed]
- Adam J, Le Stang N, Rouquette I, et al. Multicenter harmonization study for PD-L1 IHC testing in non-small-cell lung cancer. Ann Oncol 2018;29:953-8. [Crossref] [PubMed]


