Risk for cardiovascular disease in patients with nontuberculous mycobacteria treated with macrolide
Introduction
The incidence and prevalence of lung disease caused by nontuberculous mycobacteria (NTM) continue to increase around the world (1), including in South Korea (2), making NTM disease an emerging public health threat. Chronic lung infection is the most common form of NTM disease (3). In South Korea, lung disease due to NTM is most frequently caused by Mycobacterium avium complex (MAC) (3).
Patients with a history of tuberculosis are at a higher risk for developing cardiovascular disease, such as ischemic stroke or acute coronary syndrome (4,5). In addition, community-acquired pneumonia is a well-known significant risk factor for inflammation-mediated acute cardiac events (6,7). Considering that majority of patients with NTM disease can present with manifestations that are similar to those of tuberculosis or pneumonia (3,8), it is possible that NTM disease itself is a high risk factor for cardiovascular disease. Moreover, a number of studies have shown an association between increased cardiovascular morbidity and mortality with administration of macrolide (9-13), the core drug for the treatment of NTM disease (3,8). Therefore, the use of macrolide can possibly increase the risk for cardiovascular disease in patients with NTM disease.
Additionally, many studies have reported that the risk for cardiovascular disease is different between patients treated with clarithromycin and azithromycin (14-17), which are the two main macrolides used for the treatment of most NTM species (3,8). Given the similar efficacy (18) and prescription rate (19) between these two drugs, evaluation of the difference in risk for cardiovascular disease between patients treated with these two macrolides would have a clinically significant implication.
To date, no studies have examined these issues. Therefore, we aimed to investigate the incidence of acute cardiovascular events in patients with NTM disease treated with macrolide and identify whether there is a difference in the risk for cardiovascular disease between patients treated with clarithromycin and azithromycin.
Methods
Data sources and ethics
This was a population-based retrospective cohort study that used the Health Insurance Review and Assessment Service (HIRA) database of South Korea. HIRA is a government-affiliated agency that assesses the accuracy of claims for the National Health Insurance, a compulsory system that covers 96.6% of the entire population of 48.6 million, and for the National Medical Aid, which covers 3.5% of the South Korean population (20). The patients’ claims data that were submitted by healthcare providers between January 1, 2011 and December 31, 2015 were obtained. The data given by HIRA had anonymized identifiers, according to the Act on the Protection of Personal Information that is maintained by public agencies. All diagnoses were coded using the Korean Classification of Disease, sixth edition, which is a modified version of the International Classification of Disease and Related Health Problems, tenth revision (ICD-10). The database contained longitudinal patient information on demographics, diagnosis, and prescriptions. The prescription data included the drug name, dose, prescription date, and supply days.
This study protocol was approved by the Institutional Review Board of Asan Medical Center, Seoul, South Korea (IRB No. 2016-0880). Informed consent was waived because the study used an existing database that was provided in a de-identified format.
Study subjects, exposure assessment and study outcome
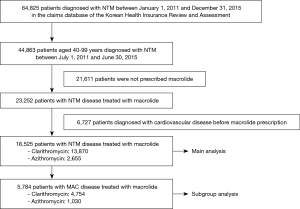
To secure at least 180 days of observation period, patients with NTM disease were chosen between July 1, 2011 and June 30, 2015 from the HIRA database. NTM disease was diagnosed in patients with claims for NTM (ICD-10 A31). Initially, a total of 44,863 patients aged 40 years or older with a diagnosis of NTM were identified from the HIRA database in the study period. Then, we excluded patients who were not prescribed macrolides and those who had been recorded to have ischemic heart disease (ICD-10 I20-5), cerebrovascular disease (ICD-10 I60-9), or cardiac arrhythmia (ICD-10 I44-5, I47-9) before the first prescription of macrolide. Patients who received both rifampin and ethambutol more than once with macrolide were defined to have MAC disease.
The use of macrolide was designated as prescription of clarithromycin or azithromycin. The index date was defined as the date of prescription of the macrolide. Based on previous studies showing a short-term use of clarithromycin was associated with increased cardiovascular mortality for as long as 10 years, (9,10), we assumed that macrolides had a persistent effect on cardiovascular disease even after discontinuation; that is, once a patient with NTM disease has been prescribed macrolide, the patient is regarded to be under the influence of it even after its discontinuation and until the end of the follow-up period (December 31, 2015) (Figure 1A). In addition, if one macrolide was subsequently changed into another, the period until the change of the first macrolide was considered as the period of use for that drug and the period of the subsequent macrolide prescription was ignored (Figure 1B). Finally, in the case of the study outcome occurred during the use or after discontinuation of the macrolide, the time of onset of the study outcome was defined as the end of the exposure duration (Figure 1C).
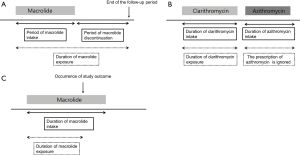
The primary outcome was the initial and subsequent hospitalization or emergency department visit for a diagnosis of cardiovascular disease along with acute myocardial infarction (ICD-10 I21-2), cerebrovascular disease (ICD-10 I60-9), and cardiac arrhythmia (ICD-10 I44-5, I47-9) (9). All patients were followed up until the study outcome occurred, a macrolide was switched, or the end of the follow-up period was reached, whichever came first.
Statistical analyses
Baseline characteristics were presented as numbers with percentages for categorical variables and as mean and standard deviation or median and interquartile range for continuous variables. The covariates selected included age at index date, gender, presence of comorbidities, and pre-existing medications. Comorbidities were identified by ICD-10, all of which were assessed for 180 days prior to the index date. We selected the comorbidities that might influence the risk for cardiovascular disease, with modified details on ICD-10 considering clinical practice (Table S1), based on Charlson index (21,22) added to known risk factors (23). The category of pre-existing medications was selected based on previous studies (11,13) (Table S2). The concomitant medications for the treatment of NTM disease were determined based on the American Thoracic Society guidelines for NTM treatment (24).
Full table

Full table
To control the potential confounders in an observational study, inverse probability treatment weights (IPTW) were applied and accounted for the limited number of cardiovascular diseases. IPTW was estimated by propensity score and was derived by logistic regression analysis for assignment to exposure groups using all baseline covariates included in Table 1; IPTW was defined as the inverse of (1 − propensity score) for clarithromycin group and as the inverse of the propensity score for the azithromycin group. In order to reduce the influence of outliers, the weights were stabilized by multiplying with the mean propensity score of the given exposure group. Balances in the distribution of the baseline covariates were estimated by the standardized difference between the two groups, before and after IPTW adjustment (25-27).
Full table
Comparison of the incidence rates of cardiovascular disease between patients with NTM disease treated with macrolide and the general population
First, we calculated the incidence rate and the 95% confidence interval (CI) of cardiovascular disease in the study subjects per 1,000 person-years assuming a Poisson distribution. Then, the standardized incidence ratio (SIR) was calculated; the observed cases were divided by the expected number of cases for each 10-year age- and gender-stratified general population in South Korea. The expected number of cases in a South Korean general population was defined as the number of subjects with cardiovascular disease in the year 2013 based on the data of the Korean National Health Insurance Service national sample cohort. This cohort comprised a representative random sample of 1,025,340 individuals, which accounts for approximately 2.2% of the entire population of South Korea (28). Byar’s approximation was used to calculate the 95% CIs for SIRs (29).
Differences in the risk for cardiovascular disease between patients treated with clarithromycin and azithromycin
Survival curves were constructed with Kaplan-Meier estimates and compared with the use of the log-rank test. The crude hazard ratio for the association between the macrolide antibiotics and the incidence of cardiovascular disease was calculated using the Cox proportional hazard model. For more rigorous control of potential confounders, the hazard ratios were adjusted based on IPTW-weighted Cox models and were further adjusted for some important covariates that might have significant effects on the outcomes. All statistical analyses were performed using the SAS Enterprise Guide software (version 6.1, SAS Institute, Inc., Cary, NC, USA).
Results
Characteristics of the study subjects
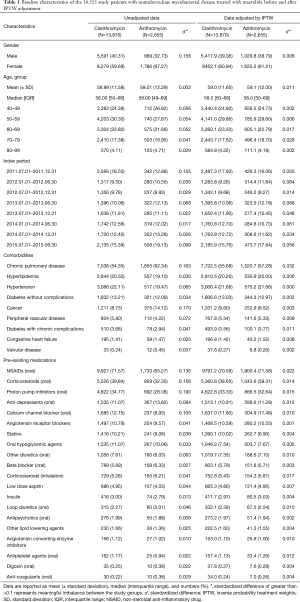
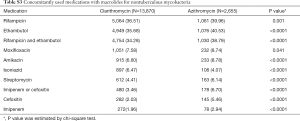
Eligibility screening identified 16,525 patients with NTM disease who received macrolide medications (Figure 2). The study population had a mean (± standard deviation) age of 59.0 (±11.9). A total of 13,870 patients were prescribed clarithromycin and the remaining 2,655 were prescribed azithromycin. The baseline characteristics of the study subjects are shown in Table 1. Among the drugs used for the treatment of NTM, rifampin and ethambutol were the most commonly prescribed drugs with macrolide. The other concomitant medications used for NTM are presented in the Table S3.
Full table
Incidence of cardiovascular disease in patients with NTM disease treated with macrolide
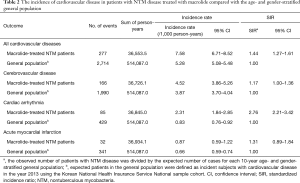
During the study period, 277 events of cardiovascular disease were identified; these included cerebrovascular disease (n=166), cardiac arrhythmia (n=85), and acute myocardial infarction (n=32). The incidence of cardiovascular disease in patients with NTM disease treated with macrolide was 7.58 per 1,000 patients per year. As shown in Table 2, the SIR of cardiovascular disease in the study population was significantly higher than that in the age- and gender-stratified general population (SIR, 1.44; 95% CI, 1.27–1.61).
Full table
Comparison of the risk for cardiovascular disease between patients treated with clarithromycin and azithromycin
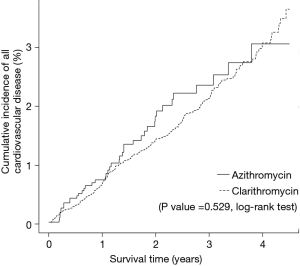
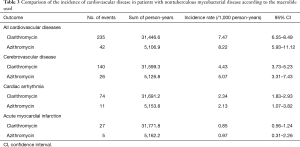
As shown in Figure 3, the risk for cardiovascular disease was not different between patients treated with clarithromycin and azithromycin in the unadjusted analysis (crude hazard ratio, 0.90; 95% CI, 0.65–1.25; Tables 3 and 4). Further analyses adjusted by IPTW alone and IPTW with the significant covariates influencing outcome likewise revealed no difference in the cardiovascular risk between patients treated with clarithromycin and azithromycin (adjusted hazard ratio, 0.91 and 0.90; 95% CI, 0.66–1.26 and 0.65–1.24, respectively; Table 4).
Full table
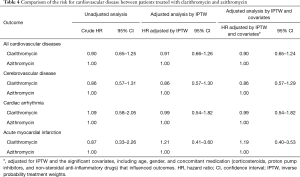
Full table
Subgroup analysis of the risk for cardiovascular disease in patients with MAC disease treated with macrolide
As shown in Figure 2, 5,784 patients were identified as having MAC disease. Subgroup analysis of these patients showed generally similar results, but SIR for cardiovascular disease was higher than that of NTM disease compared with general population (SIR, 1.96; 95% CI, 1.65–2.30) (Table S4). In addition, we found no difference in the risks for cardiovascular disease between patients with MAC disease treated with clarithromycin and those treated with azithromycin (Table S5), although this interpretation may be biased because of the small number of cardiovascular events in this subgroup of patients.
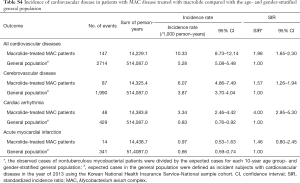
Full table

Full table
Discussion
NTM disease can lead to various complications, such as substantial decline in lung function (30), respiratory failure (31), and mortality (32). However, the level of risk for cardiovascular disease in patients with NTM disease treated with macrolide is unknown. To the best of our knowledge, this was the first study that aimed to assess this issue. Based on our analyses, the most important finding was the significantly higher risk for cardiovascular disease in NTM patients aged 40 years or older treated with macrolide than in the age- and gender-stratified general population. This finding was more evident in patients with MAC disease. Further, the increased risk for developing cardiovascular disease was not different between patients treated with clarithromycin and azithromycin.
There are several plausible explanations for the increased incidence of cardiovascular disease in patients with NTM disease treated with macrolide. First, the inability to eradicate NTM from the lungs can lead to an exuberant inflammatory response that is modulated by the immune and airway epithelial cells through the release of inflammatory cytokines (33). This condition can result in endothelial dysfunction and the exposure/release of factor VII, tissue factor, von Willebrand factor, and subendothelial collagen, creating a pro-coagulant state (6) that can lead to acute cardiovascular events. Second, the increased cardiovascular risk may be associated with bronchiectasis which is frequently found in patients with NTM lung disease (24). Bronchiectasis is known as a predisposing factor for NTM infection; conversely, NTM infection can lead to development of bronchiectasis (33). Of note, patients with bronchiectasis have an increased cardiovascular risk, possibly due to systemic inflammation, that may contribute to the development of atherosclerosis (34). Considering that the most common form of NTM lung disease is a nodular bronchiectatic type (35) and given the close association between bronchiectasis and NTM lung disease, bronchiectasis may be a link between NTM lung disease and acute cardiovascular events. Third, macrolides accumulate and promote the growth of macrophages, which results in plaque formation due to thrombosis (10,36). Fourth, because of the potassium channel blocking properties, macrolides are known to prolong the QT interval and increase the risk for arrhythmias (13,37). Arrhythmia may also play a role in plaque rupture that leads to cardiovascular events (11). However, given the lack of experimental evidence to support specific mechanisms behind the association between macrolide antibiotics and cardiovascular risk in NTM lung disease, future studies are needed to elucidate the mechanisms explaining this finding.
A number of studies that compared the cardiovascular disease risk between clarithromycin and azithromycin showed conflicting results. For example, a study by Chou et al. in Taiwan reported that azithromycin was associated with a significant increase in the risks for ventricular arrhythmia and cardiovascular death compared with amoxicillin–clavulanate; in contrast, clarithromycin and ciprofloxacin were not associated with adverse cardiac outcomes (14). In animal studies, clarithromycin was demonstrated to have more prominent proarrhythmic potential than azithromycin (15,16). Meanwhile, one study in Canada showed no significant differences between patients treated with clarithromycin and azithromycin in terms of 12 composite outcomes, including acute myocardial infarction, arrhythmia, and ischemic stroke (17). In the present study, no difference in the risk for cardiovascular disease was found between patients treated with clarithromycin and azithromycin.
The use of macrolide has been demonstrated beneficial in several respiratory diseases due to its anti-inflammatory effect (38-40). However, we found a higher cardiovascular risk in patients treated with macrolides than in the general population. Two lines of reasoning could explain this result. First, the impact of macrolides on the cardiovascular risk may have been stronger than the anti-inflammatory effects in patients with NTM lung disease. Second, NTM lung disease itself may have led to a significantly higher incidence of cardiovascular disease despite the macrolide anti-inflammatory effects, resulting in a higher risk in patients with NTM lung disease than that in the general population. Although at the moment it is impossible to understand how or why the anti-inflammatory effects of macrolides were diminished in the present study, our result is still useful to physicians because the increased acute cardiovascular events risk among this population suggests that close patient monitoring and proper preventive strategies may be necessary.
We assumed that the effects of the macrolide were prolonged even after discontinuing the drug, but one study suggested that cardiovascular disease immediately developed within a few days after drug intake and did not persist after the course of therapy ended (13). The design of the present study did not allow precise assessment of this short-term effect of macrolides on cardiovascular disease. Accordingly, we performed a subgroup analysis for patients with MAC disease with the assumption that the effects of macrolide disappeared soon after discontinuing the drug, but within a designated grace period (additional methods is provided in the Supplementary Data). The results of this analysis were generally the same as the results of the analysis of the overall study population (see Tables S6,S7).
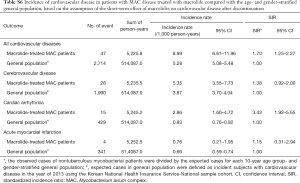
Full table
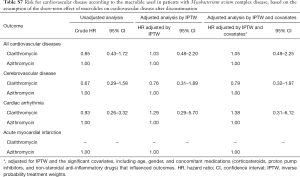
Full table
Our current study had several limitations. Most importantly, the diagnoses of NTM disease and the prescription of macrolide were solely dependent on the HIRA claims data, which were less accurate than the diagnoses made according to standardized criteria. Second, use of the HIRA data did not enable us to exclude patients with NTM disease other than lung infections, such as lymphadenopathy and skin and soft tissue infections. Third, although one of the important results of our study was the more pronounced risk for cardiovascular disease in MAC disease than in NTM disease, the definition of MAC disease may not have been accurate. That is, our definition of MAC disease based on a combination of rifampin and ethambutol with macrolide can also be used in the other common NTM species, such as Mycobacterium kansasii (41). However, M. kansasii has been shown to represent only 1–2% of all NTM isolates and pathogens in NTM lung disease in South Korea (41). Fourth, we failed to include the body mass index and the lifestyle factors, such as alcohol consumption, smoking, and physical activity, which are known to influence the risk for cardiovascular disease. Lastly, in this study based on HIRA data, we could not tell whether the increased cardiovascular risk was due to the NTM disease itself, to the macrolides, or to both. We had to exclude patients identified with ICD-10 codes for NTM without macrolide treatment, because it was unclear whether they actually met the ATS/IDSA NTM lung disease diagnostic criteria. We only included data from patients with macrolide prescriptions. However, it should be noted as a limitation that the macrolides may have been used to treat conditions other than NTM disease (e.g., pneumonia).
In conclusion, the incidence of cardiovascular disease was significantly higher in patients aged 40 years or older treated with macrolide for nontuberculous mycobacterial disease than in the general population. This risk was not different between patients treated with clarithromycin and azithromycin.
Supplementary
Additional methods
Because previous study have shown that the cardiovascular side effects of macrolides developed within several days of administration, we set a grace period based on the assumption that the effects of the macrolides would resolve soon after discontinuation of the drug. We calculated the duration from initiation to discontinuation of macrolide use based on the number of days between refills. In cases in which the macrolide was prescribed for less than 30 days, we allowed a 30-day maximum permissible refill gap of the prescription between the end of the latest prescription and the date of the subsequent dose. In cases in which the macrolide was prescribed for more than 30 days, twice the prescribed period plus 30 days was the designated permissible refill gap. The other methods, definition of other exposure assessments, occurrence of outcome, and switch to another macrolide were the same as those of the original analysis.
Acknowledgements
We thank the Korean Health Insurance Review and Assessment Service and the National Health Insurance Service for providing the insurance claims data.
Funding: This research was supported by the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea (grant number: 2016–617).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study protocol was approved by the Institutional Review Board of Asan Medical Center, Seoul, South Korea (IRB No. 2016-0880). Informed consent was waived because the study used an existing database that was provided in a de-identified format.
References
- Brode SK, Daley CL, Marras TK. The epidemiologic relationship between tuberculosis and non-tuberculous mycobacterial disease: a systematic review. Int J Tuberc Lung Dis 2014;18:1370-7. [Crossref] [PubMed]
- Yoo JW, Jo KW, Kim MN, et al. Increasing trend of isolation of non-tuberculous mycobacteria in a tertiary university hospital in South Korea. Tuberc Respir Dis (Seoul) 2012;72:409-15. [Crossref] [PubMed]
- Ryu YJ, Koh WJ, Daley CL. Diagnosis and Treatment of Nontuberculous Mycobacterial Lung Disease: Clinicians' Perspectives. Tuberc Respir Dis (Seoul) 2016;79:74-84. [Crossref] [PubMed]
- Sheu JJ, Chiou HY, Kang JH, et al. Tuberculosis and the risk of ischemic stroke: a 3-year follow-up study. Stroke 2010;41:244-9. [Crossref] [PubMed]
- Chung WS, Lin CL, Hung CT, et al. Tuberculosis increases the subsequent risk of acute coronary syndrome: a nationwide population-based cohort study. Int J Tuberc Lung Dis 2014;18:79-83. [Crossref] [PubMed]
- Feldman C, Anderson R. Community-Acquired Pneumonia: Pathogenesis of Acute Cardiac Events and Potential Adjunctive Therapies. Chest 2015;148:523-32. [Crossref] [PubMed]
- Corrales-Medina VF, Musher DM, Shachkina S, et al. Acute pneumonia and the cardiovascular system. Lancet 2013;381:496-505. [Crossref] [PubMed]
- Kwon YS, Koh WJ. Diagnosis and Treatment of Nontuberculous Mycobacterial Lung Disease. J Korean Med Sci 2016;31:649-59. [Crossref] [PubMed]
- Schembri S, Williamson PA, Short PM, et al. Cardiovascular events after clarithromycin use in lower respiratory tract infections: analysis of two prospective cohort studies. Bmj. 2013;346:f1235. [Crossref] [PubMed]
- Winkel P, Hilden J, Hansen JF, et al. Clarithromycin for stable coronary heart disease increases all-cause and cardiovascular mortality and cerebrovascular morbidity over 10years in the CLARICOR randomised, blinded clinical trial. Int J Cardiol 2015;182:459-65. [Crossref] [PubMed]
- Wong AY, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ 2016;352:h6926. [Crossref] [PubMed]
- Svanström H, Pasternak B, Hviid A. Use of clarithromycin and roxithromycin and risk of cardiac death: cohort study. BMJ 2014;349:g4930. [Crossref] [PubMed]
- Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012;366:1881-90. [Crossref] [PubMed]
- Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and beta-lactam/beta-lactamase inhibitors: a Taiwanese nationwide study. Clin Infect Dis 2015;60:566-77. [Crossref] [PubMed]
- Milberg P, Eckardt L, Bruns HJ, et al. Divergent proarrhythmic potential of macrolide antibiotics despite similar QT prolongation: fast phase 3 repolarization prevents early afterdepolarizations and torsade de pointes. J Pharmacol Exp Ther 2002;303:218-25. [Crossref] [PubMed]
- Ohtani H, Taninaka C, Hanada E, et al. Comparative pharmacodynamic analysis of Q-T interval prolongation induced by the macrolides clarithromycin, roxithromycin, and azithromycin in rats. Antimicrob Agents Chemother 2000;44:2630-7. [Crossref] [PubMed]
- Fleet JL, Shariff SZ, Bailey DG, et al. Comparing two types of macrolide antibiotics for the purpose of assessing population-based drug interactions. BMJ Open 2013;3. [Crossref] [PubMed]
- Wallace RJ Jr, Brown-Elliott BA, McNulty S, et al. Macrolide/Azalide therapy for nodular/bronchiectatic mycobacterium avium complex lung disease. Chest 2014;146:276-82. [Crossref] [PubMed]
- Adjemian J, Prevots DR, Gallagher J, et al. Lack of adherence to evidence-based treatment guidelines for nontuberculous mycobacterial lung disease. Ann Am Thorac Soc 2014;11:9-16. [Crossref] [PubMed]
- Kim DS. Introduction: health of the health care system in Korea. Soc Work Public Health 2010;25:127-41. [Crossref] [PubMed]
- Charlson ME, Sax FL, MacKenzie CR, et al. Morbidity during hospitalization: can we predict it? J Chronic Dis 1987;40:705-12. [Crossref] [PubMed]
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130-9. [Crossref] [PubMed]
- Lin GA, Dudley RA, Lucas FL, et al. Frequency of stress testing to document ischemia prior to elective percutaneous coronary intervention. JAMA 2008;300:1765-73. [Crossref] [PubMed]
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367-416. [Crossref] [PubMed]
- Stürmer T, Wyss R, Glynn RJ, et al. Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental study designs. J Intern Med 2014;275:570-80. [Crossref] [PubMed]
- Brookhart MA, Wyss R, Layton JB, et al. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes 2013;6:604-11. [Crossref] [PubMed]
- Lanehart RE dGP, Kim ES, Bellara AP, et al. Propensity score analysis and assessment of propensity score approaches using SAS procedures. SAS Global Forum 2012, Statistics and Data Analysis.
- Lee J, Lee JS, Park SH, et al. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol 2017;46. [PubMed]
- Breslow NE, Day NE. Statistical methods in cancer research. Volume II--The design and analysis of cohort studies. IARC Sci Publ 1987.1-406. [PubMed]
- Park HY, Jeong BH, Chon HR, et al. Lung Function Decline According to Clinical Course in Nontuberculous Mycobacterial Lung Disease. Chest 2016;150:1222-32. [Crossref] [PubMed]
- Yeh JJ, Wang YC, Lin CL, et al. Nontuberculous mycobacterial infection is associated with increased respiratory failure: a nationwide cohort study. PLoS One 2014;9. [Crossref] [PubMed]
- Hwang JA, Kim S, Jo KW, et al. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur Respir J 2017;49. [Crossref] [PubMed]
- Honda JR, Knight V, Chan ED. Pathogenesis and risk factors for nontuberculous mycobacterial lung disease. Clin Chest Med 2015;36:1-11. [Crossref] [PubMed]
- Navaratnam V, Millett ER, Hurst JR, et al. Bronchiectasis and the risk of cardiovascular disease: a population-based study. Thorax 2017;72:161-6. [Crossref] [PubMed]
- Hayashi M, Takayanagi N, Kanauchi T, et al. Prognostic factors of 634 HIV-negative patients with Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2012;185:575-83. [Crossref] [PubMed]
- Robbins CS, Hilgendorf I, Weber GF, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med 2013;19:1166-72. [Crossref] [PubMed]
- Straus SM, Sturkenboom MC, Bleumink GS, et al. Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur Heart J 2005;26:2007-12. [Crossref] [PubMed]
- Garin N, Genne D, Carballo S, et al. beta-Lactam monotherapy vs beta-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: a randomized noninferiority trial. JAMA Intern Med 2014;174:1894-901. [Crossref] [PubMed]
- Altenburg J, de Graaff CS, Stienstra Y, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 2013;309:1251-9. [Crossref] [PubMed]
- Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011;365:689-98. [Crossref] [PubMed]
- Moon SM, Park HY, Jeon K, et al. Clinical Significance of Mycobacterium kansasii Isolates from Respiratory Specimens. PLoS One 2015;10. [Crossref] [PubMed]