The performance and limitation of T-SPOT.TB for the diagnosis of TB in a high prevalence setting
Introduction
Tuberculosis (TB) poses a great global threaten to human health. According to the report of the World Health Organization, in 2011, there were an estimated 8.7 million new cases of TB [13% co-infected with human immunoddficiency virus (HIV)] and 1.4 million people died from TB (1). The difficulty in diagnosis hampers the progress of prevention and control for TB. As a classical immunoassay, the tuberculin skin test (TST) has been widely used in the diagnosis of TB for a long time, but the method was found in a poor specificity and accuracy, mainly due to the cross-reactions of tuberculin purified protein derivatives (PPD) with that induced by BCG (2-4). Recently, the T-SPOT.TB assay has shown to be higher performance for diagnosis of TB. The T-SPOT.TB assay is a simplified enzyme-linked immunospot (ELISPOT) method, which is designed for the detection of effector T cells that respond to stimulation by specific antigens [early secreted antigenic target 6 kDa (ESAT-6) and culture filtrate protein 10 kDa (CFP10)] for Mycobacterium tuberculosis (MTB) (3,5-9). ESAT-6 and CFP10 only present in MTB but absent in bacille Calmette-Guerin (BCG) strains, which ensure that the T-SPOT.TB assay could be a high specificity theoretically. The previous reports have shown that the T-SPOT.TB assay may be a more accurate indicator of the presence of LTBI and active TB (ATB), however, most of these studies occurred in the low prevalence settings (10-14). Therefore, it is significant to evaluate the diagnostic value of T-SPOT.TB for the specific populations in a high prevalence setting. In this present study, we conducted stratified and comparable analyses to explore the clinical value and the limitation of T-SPOT.TB assay for TB diagnosis in a high TB prevalence setting, China, one of the 22 high-burden countries (1).
Study populations and methods
Ethical statement
All participants were treated in accordance with the Declaration of Helsinki on the participation of human subjects in medical research. Written informed consent was obtained from each of subjects and the study was approved by the Ethics Committee of Shanghai Pulmonary Hospital.
Study population
A total of 413 subjects were included in the study. The subjects were divided into four groups including ATB group, non-TB pulmonary diseases (NTBPDs) group, medical staff group and healthy controls group. Both the ATB group and the NTBPDs group were randomly selected from the inpatients attending Shanghai Pulmonary Hospital between January 2005 and December 2008, including 202 cases of ATB patients [163 cases of pulmonary TB (PTB) plus 39 cases of extrapulmonary TB (EPTB)] and 106 cases of NTBPDs patients, respectively. In addition, 20 medical staff from Department of Tuberculosis, Shanghai Pulmonary Hospital and 85 healthy volunteers were also enrolled in this study. All subjects were shown to be negative by HIV antibody testing. Amongst ATB group, there were found in 30 cases of patients combined with diabetes mellitus (DM). However, there had no drop-out subjects in this study. The general characteristics included in this study are shown in Table 1.
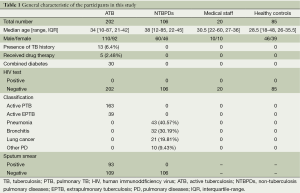
Full table
A definite diagnosis of ATB, PTB, and EPTB was made according to the criteria by Chinese Antituberculosis Association, mainly relying on signs and symptoms, an X-ray, computer tomography (CT) and identifying MTB in a clinical sample (sputum or a tissue biopsy). In detail, the definite diagnose of ATB was made on as following: (I) ‘culture/biopsy-confirmed ATB’ relied on the positive culture or smear of MTB from sputum or biopsy specimen; (II) ‘clinical ATB’ lack of bacterial/pathological evidence was based on clinical manifest or radiographic responses to anti-TB treatment. NTBLDs are the patients who had no past history of TB, including pneumonia, bronchitis, lung carcinoma and other lung diseases. The diagnostic criteria for smear negative PTB is as follows: (I) had typical clinical symptoms of PTB and identical chest X-ray manifestations; (II) recieved a positive effect with anti-TB treatment; (III) excluded other non-tuberculous PD; (IV) presented positive serum test; (V) had a positive result by PCR for the detection of sputum sample; (VI) the pathology of lung tissue confirmed TB; (VII) detected a positive mycobacterium in bronchoalveolar lavage fluid; (VIII) a tuberculous-like lesion was confirmed in the bronchial and lung tissue by pathology examination. It was confirmed if there had three clauses in former six clauses or one of 7th and 8th clauses. The diagnosis of NTBLDs was made on the bacteriology, clinical manifest and other checks. NTBPDs and DM were independently diagnosed by professional clinicians based on the relevant medicine evidence. Besides, the laboratory staff was blinded to the clinical diagnosis of the subjects when they performed the tests in this present study.
T-SPOT.TB assay
T-SPOT.TB assay was performed following the instructions of the assay kit (Oxford Immunote Ltd., Edinburgh, UK). The procedure is described as following. Peripheral blood mononuclear cells (PBMCs) is separated from a whole blood sample and washed to remove any sources of background interfering signal. Four wells including a nil control, panel A (ESAT-6), panel B (CFP10), and a positive control containing phytohaemagglutinin is required for each sample. The PBMCs is incubated with the antigens and the secreted cytokine by sensitized T cell is captured by specific antibodies on the membrane, and then the cells and other unwanted materials are removed by washing. Finally, the cytokine was detected by a chromogenic spot assay. The evaluation of the testing result is interpreted by spot count, according to the following algorithm: (I) the test result is ‘Positive’ if (panel A minus nil control) and/or (panel B minus nil control) ≥6 spots; and (II) the test result is ‘Negative’ if both (panel A minus nil control) and (panel B minus nil control) ≤5 spots. This includes values less than zero.
Statistical analysis
Difference in categorical variables was evaluated using chi-square Test. The statistical analyses were performed with the SPSS 13.0 (SPSS Inc., Chicago, Illinois, USA). A P value of less than 0.05 was considered significant.
Results
Infection risk of TB in the PTB, NTBPDs, medical staff and healthy controls
According to the testing results of T-SPOT.TB, the positive rates in the ATB, NTBPDs, medical staff, and healthy controls were 85.64%, 33.96%, 30.00% and 8.24%, respectively. The T-SPOT positive incidence in NTBPDs and medical staff was significantly higher than that in the healthy controls (Table 2).

Full table
The performance of T-SPOT.TB for the diagnosis of ATB
Compared with the healthy controls, the sensitivity, specificity, likelihood ratio positive (+LR), likelihood ratio negative (–LR), and diagnostic odd ratio (DOR) of T-SPOT.TB for the diagnosis of ATB were 85.64%, 91.76%, 10.40%, 0.16%, and 66.47%, respectively (Table 3). These results were shown to be a higher performance in the diagnosis of ATB in a healthy population, but not perfect.

Full table
When compared with the NTBPDs, the sensitivity, specificity, +LR and –LR, and DOR of T-SPOT.TB for the diagnosis of ATB were 85.64%, 66.04%, 2.52%, 0.22%, and 11.6%, respectively (Table 4). The specificity, +LR, and DOR were significantly lower than those in Table 3 (P<0.05), indicating that T-SPOT.TB had a lower performance in the diagnosis of ATB in the NTBPDs population.

Full table
Subgroup analyses of the sensitivities of T-SPOT.TB in the diagnosis of ATB
Both APTB group and EPTB group were shown to have high sensitivities. No significant difference was found in the sensitivities of T-SPOT.TB between two groups (Table 5). Subgroup analysis revealed that the sensitivity of T-SPOT.TB in the diagnosis of ATB wasn’t subjected to diabetes (Table 6). According to the testing results stratified by sputum smear microscopy, the sensitivity in sputum smear-positive group was significantly higher than that in smear-negative group (Table 7).

Full table

Full table

Full table
Discussion
As a promising approach for the diagnosis of ATB and latent TB infection (LTBI), it is inspiring of the clinical application of the interferon-gamma release assays (IGRAs) including QuantiFERON TB Gold (QFT-G; Cellestis, Ltd., Carnegie, Australia) and T-SOPT.TB. However, for T-SOPT.TB, there still need to accumulate clinical evidences in different settings and populations to validate its potential values and limitations. In this study, we investigated the TB infection in the populations including ATB, NTBPDs, medical staff, and healthy controls by T-SOPT.TB assay.
According to the results of T-SPOT.TB, the positive rates in the NTBPDs, medical staff, and healthy controls were 33.96%, 30.00% and 8.24%, respectively. This demonstrates that the healthy controls in Southern China may be a high incidence of LTBI. Surprisingly, there were found in more than 30% suspected LTBI in the NTBPDs and medical staff. We postulates that the increasing expose factor in medical settings may play an important role in this outcome, which suggests that it is urgent and important to improve the personal safety protection in the hospital setting of high prevalence area.
When compared with a low risk of TB infection population (the healthy controls), the sensitivity, specificity, +LR, –LR, and DOR of T-SPOT.TB for the diagnosis of ATB were 85.64%, 91.76%, 10.40%, 0.16%, and 66.47%, respectively. It suggests that the T-SPOT.TB had a higher performance in the diagnosis of ATB in a lower risk of TB infection population. In contrast, the previous studies in low prevalence setting revealed that the T-SPOT.TB had a very high specificity (90-100%) (11,13,15).
However, when compared with a high risk of TB infection population (the NTBPDs controls), the sensitivity, specificity, +LR, –LR, and DOR of T-SPOT.TB for the diagnosis of ATB were 85.64%, 66.04%, 2.52%, 0.22%, and 11.6%, respectively. The specificity, +LR, and DOR were lower, indicating T-SPOT.TB had a lower performance (especially for specificity) in the diagnosis of ATB in a high risk of TB infection population. Therefore, we believed that the performance of T-SPOT.TB for the diagnosis of ATB in a high risk of TB infection population isn’t as good as that in a low risk of TB infection population, and that the high incidences of LTBI interfere in the diagnosis of ATB. So, it need be cautious for interpreting the testing results of T-SPOT.TB for the diagnosis of ATB in the high risk of TB infection populations such as the patients with PD or medical staff or close contacts. Under this condition, the value of rule-out is seemed to be better than that of rule-in.
According to a meta-analysis in 2011 (16), the specificity of the IGRAs for the diagnosis of LTBI, IGRAs varied 98-100%. IGRAs positivity was clearly associated with exposure to contagious TB cases. In conclusion, IGRAs may have a relative advantage over the TST in detecting LTBI and allow the exclusion of TB infection with higher reliability. Another meta-analysis including a combined sample size of 26,680 participants revealed that IGRAs didn’t have high accuracy for the prediction of ATB, although use of IGRAs in some populations might reduce the number of people considered for preventive treatment (17). However, because that these meta-analysis didn’t conduct subgroup analyses of specific populations, which is different from our findings. Our study revealed that IGRAs may have the diagnostic value of rule-out in a high risk of TB infection population; however, our study also demonstrated that IGRAs for the diagnosis of ATB in a low risk of TB infection population had high accuracy and DOR. However, according to an updated meta-analysis of the diagnostic accuracy of IGRAs for TB disease (18), the overall sensitivity, specificity, +LR, –LR and DOR of IGRAs were 0.85 (95% CI: 0.84-0.86), 0.84 (95% CI: 0.83-0.85), 7.82 (95% CI: 6.01-10.19), 0.17 (95% CI: 0.14-0.21), and 59.27 (95% CI: 40.19-87.42), respectively. For ten studies evaluating T-SPOT TB in China, the combined sensitivity, specificity, +LR, –LR and DOR were 0.88 (95% CI: 0.86-0.91), 0.89 (95% CI: 0.86-0.92), 8.86 (95% CI: 5.42-14.46), 0.13 (95% CI: 0.10-0.17), and 88.15 (95% CI: 41.76-186.07), respectively. Compared with this meta-analysis, the specificity, +LR and DOR of IGRAs for detecting ATB in a high risk of TB infection population in this study is significantly lower. However, there had no difference in the sensitivities between two studies.
In this study, we also conducted the subgroup analyses of the sensitivities of T-SPOT.TB for the diagnosis of ATB according to PTB and EPTB, with/without DM, and sputum smear-positive and smear-negative. We found that both active PTB group and EPTB group were shown to have the higher sensitivities. No significant difference was found in the sensitivity between two groups. Given that the diagnosis of EPTB is very difficult at present, it is valuable that the saving time assay achieves such a high sensitivity. In this study, there were 30 cases of ATB patients with DM. In order to clarify whether the alternation of immune status induced by DM interferes in the T-SPOT.TB sensitivity or not, we performed a subgroup analysis for the DM group and NDM group. The results revealed that the sensitivity of T-SPOT.TB for the diagnosis of ATB wasn’t subjected to diabetes, which indicates that T-SPOT.TB is also suitable for screening of ATB with DM.
We found that the T-SPOT.TB sensitivity for diagnosis of ATB in sputum smear-positive group was significantly higher than that in smear-negative group. We postulate that TB bacilli numbers is likely to affect the T-SPOT.TB. The previous study showed that IFN-gamma-producing RD1-specific T cells, as measured in the T-SPOT.TB assay, may be directly related to bacterial load in patients undergoing treatment for PTB (19). The study included in 491 smear-negative children from two hospitals in Cape Town, South Africa revealed that in a high-burden setting, the T-SPOT.TB did not have added value beyond clinical data and conventional tests for diagnosis of TB disease in smear-negative children (20). However, it needs a further study to confirm.
It must be pointed out this study had some limitations. The sample numbers of EPTB, DM, and medical staff is small, which may result in low validations of evidence. Additionally, due to the lack of the “gold standard” of LTBI diagnosis (21,22), we couldn’t evaluate the complete performance of T-SPOT.TB for the diagnosis of LTBI.
According to T-SPOT.TB, there has a high incidence of LTBI in the general population in Southern China, especially in the NTBPDs and medical staff. The T-SPOT.TB had a higher performance in the diagnosis of ATB in a low risk of TB infection population, but the T-SPOT.TB for diagnosis of ATB in the high risk of TB infection populations involving close contacts such as the patients with PD or medical staff isn’t reliable due to the interference by LTBI. T-SPOT.TB is suitable for screening the PTB, EPTB and the ATB combined with DM. However, the T-SPOT.TB sensitivity in sputum smear-negative population isn’t as high as that in smear-positive population. Therefore, we believe that the T-SPOT.TB testing results should be interpreted with caution combined with subject’s characteristics in a high prevalence setting.
Conclusions
The T-SPOT.TB assay for the diagnosis of TB has some limitations in a high prevalence setting and its clinical significance should be interpreted with caution combined with subject’s characteristics.
Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program, no. 2012CB518706), the National Natural Science Foundation of China (no. 81201253), the Science and Technology Bureau of Changzhou Municipality (no. CJ2012202), the Preventive Medicine Project of the Provincial Public Health Bureau of Jiangsu (no. Y2012095) and the Shanghai Municipal Health Bureau (no. 20124200).
Disclosure: The authors declare no conflict of interest.
References
- World Health Organization. Country profiles. Global tuberculosis report 2012.
- Ramos JM, Robledano C, Masiá M, et al. Contribution of interferon gamma release assays testing to the diagnosis of latent tuberculosis infection in HIV-infected patients: a comparison of QuantiFERON-TB Gold In Tube, T-SPOT.TB and tuberculin skin test. BMC Infect Dis 2012;12:169. [PubMed]
- Zhou L, Shen S, He M, et al. T-SPOT.TB in the diagnosis of tuberculous peritonitis. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2013;38:526-31. [PubMed]
- Lee YM, Park KH, Kim SM, et al. Risk factors for false-negative results of T-SPOT.TB and tuberculin skin test in extrapulmonary tuberculosis. Infection 2013;41:1089-95. [PubMed]
- Lalvani A, Nagvenkar P, Udwadia Z, et al. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J Infect Dis 2001;183:469-77. [PubMed]
- Pathan AA, Wilkinson KA, Klenerman P, et al. Direct ex vivo analysis of antigen-specific IFN-gamma-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J Immunol 2001;167:5217-25. [PubMed]
- Lalvani A, Pathan AA, Durkan H, et al. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet 2001;357:2017-21. [PubMed]
- Lalvani A, Pathan AA, McShane H, et al. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am J Respir Crit Care Med 2001;163:824-8. [PubMed]
- Zhao J, Wang Y, Wang H, et al. Low agreement between the T-SPOT®.TB assay and the tuberculin skin test among college students in China. Int J Tuberc Lung Dis 2011;15:134-6. [PubMed]
- Sultan B, Benn P, Mahungu T, et al. Comparison of two interferon-gamma release assays (QuantiFERON-TB Gold In-Tube and T-SPOT.TB) in testing for latent tuberculosis infection among HIV-infected adults. Int J STD AIDS 2013;24:775-9. [PubMed]
- Talbot EA, Harland D, Wieland-Alter W, et al. Specificity of the tuberculin skin test and the T-SPOT.TB assay among students in a low-tuberculosis incidence setting. J Am Coll Health 2012;60:94-6. [PubMed]
- Bienek DR, Chang CK. Evaluation of an interferon-gamma release assay, T-SPOT.TB, in a population with a low prevalence of tuberculosis. Int J Tuberc Lung Dis 2009;13:1416-21. [PubMed]
- Barsegian V, Mathias KD, Wrighton-Smith P, et al. Prevalence of latent tuberculosis infection in German radiologists. J Hosp Infect 2008;69:69-76. [PubMed]
- Cheallaigh CN, Fitzgerald I, Grace J, et al. Interferon gamma release assays for the diagnosis of latent TB infection in HIV-infected individuals in a low TB burden country. PLoS One 2013;8:e53330. [PubMed]
- Adams LV, Waddell RD, Von Reyn CF. T-SPOT.TB Test(R) results in adults with Mycobacterium avium complex pulmonary disease. Scand J Infect Dis 2008;40:196-203. [PubMed]
- Diel R, Goletti D, Ferrara G, et al. Interferon-γ release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. Eur Respir J 2011;37:88-99. [PubMed]
- Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-γ release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2012;12:45-55. [PubMed]
- Dai Y, Feng Y, Xu R, et al. Evaluation of interferon-gamma release assays for the diagnosis of tuberculosis: an updated meta-analysis. Eur J Clin Microbiol Infect Dis 2012;31:3127-37. [PubMed]
- Ribeiro S, Dooley K, Hackman J, et al. T-SPOT.TB responses during treatment of pulmonary tuberculosis. BMC Infect Dis 2009;9:23. [PubMed]
- Ling DI, Nicol MP, Pai M, et al. Incremental value of T-SPOT.TB for diagnosis of active pulmonary tuberculosis in children in a high-burden setting: a multivariable analysis. Thorax 2013;68:860-6. [PubMed]
- Lagrange PH, Simonney N, Herrmann JL. New immunological tests in the diagnosis of tuberculosis. Rev Mal Respir 2007;24:453-72. [PubMed]
- Moczko J, Słomko Z, Breborowicz G. Mathematical foundations for biophysical methods in fetal monitoring. II. Cardiotocography. Ginekol Pol 1989;60:343-9. [PubMed]


