Risk stratification of severe aortic stenosis according to new guidelines: long term outcomes
Introduction
Aortic valve stenosis (AVS) is recognized as the most prevalent valvular disease in Western countries. In patients with severe AVS, survival decreases significantly after the onset of symptoms and is usually less than 2 to 3 years (1-4). The timing of surgical treatment for severe AVS is currently guided by clinical and/or echocardiographic evaluation. ESC/EACTS 2017 Guidelines and the AHA/ACC 2017 Focused Update of the 2014 ACC/AHA Guidelines (5,6) recommend aortic valve replacement (AVR) with a class I indication in patients with symptomatic severe AVS and in asymptomatic patients with reduced left ventricular ejection fraction (LVEF <50%) or positive exercise tests. However, the management of patients with asymptomatic severe AVS and LVEF >50% without a positive stress test is still a matter of debate and the indication for surgery according to international guidelines is class IIa.
According to some authors (7,8), early surgery is not justified in asymptomatic patients with LVEF >50% because their long-term outcomes after AVR are not better than those for symptomatic patients with LVEF >50%. Alternatively, the results of other studies support early surgery for asymptomatic severe AVS based on improvement in echocardiographic parameters only (valve area index, flow-gradient patterns, calcium score) (9,10).
The purpose of our study was to examine the long-term outcomes of a large cohort of patients who underwent AVR for severe aortic valve stenosis on our institution over an 11-year period and to evaluate their indication to surgery compared to international guidelines.
Methods
We retrospectively analyzed the medical records of patients who had undergone AVR for isolated severe AVS at our institution from January 2001 to December 2012. All patients had undergone isolated AVR with both mechanical and biological prosthesis. Patients were excluded if they (I) presented concomitant severe aortic regurgitation (AR); (II) underwent emergency AVR; or (III) did not have long-term follow-up (FU), owing to death within 30 days. Clinical and echocardiographic data were collected preoperatively and at 30-day and >1-year FU examinations.
The study population was divided into groups according to symptoms based on New York Heart Association (NYHA) functional classification (class =I or ≥II) as well as preoperative ventricular function (LVEF < or ≥50%). Thus, four different subgroups were identified: group A: asymptomatic patients with preserved LVEF (NYHA =I, LVEF >50%); group B: asymptomatic patients with LV dysfunction (NYHA =I, LVEF <50%); group C: symptomatic patients with preserved LVEF (NYHA ≥II, LVEF ≥50%); group D: symptomatic patients with LV dysfunction (NYHA ≥II, LVEF <50%).
All patients signed the Institutional informed written consent before intervention authorizing Institutional Investigators to collect their clinical anonymized data and use them for scientific purposes only. Institutional review board evaluation was waived based on the previous statement and on the retrospective design of the study.
Statistical analysis
Continuous variables were compared between groups by means of a t-test or Mann-Whitney test. For categorical variables, a Fisher’s test or a chi-square test was used. Multivariate analysis of independent risk factors for mortality was carried out by means of a Cox model and survival was compared between subgroups using a logrank test; hazard ratios (HR) are reported with 95% confidence intervals. The level of statistical significance was standardized at a P<0.05. Mortality was estimated by means of a Kaplan-Meier curve.
Results
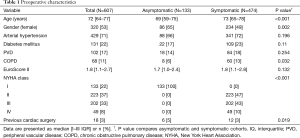
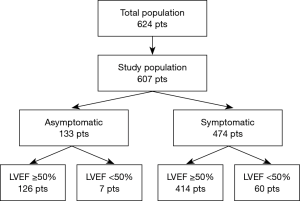
Six hundred and twenty-four patients underwent isolated AVR for severe AVS at our institution. Seventeen patients were excluded from the analysis: 12 (1.8%) died within 30 days and 5 were treated in emergency (0.8%). The remaining 607 patients constituted the study population. One hundred and thirty-three patients were asymptomatic (22%), while 474 were symptomatic (78%). Symptomatic patients were older (73 vs. 69 years, P<0.001), more frequently male (51% vs. 35%, P=0.002), and had a higher incidences of chronic obstructive pulmonary disease (COPD) (13% vs. 6%, P=0.032) and LV dysfunction (13% vs. 5%, P=0.019). Complete preoperative clinical and echocardiographic characteristics of the study population, as well as symptomatic and asymptomatic subgroups, are shown in Tables 1 and 2, respectively.
Full table
Full table
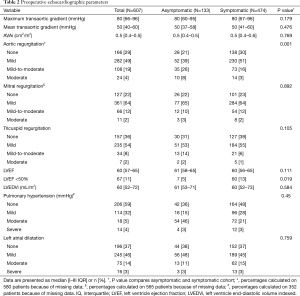
Further division of the study population by LV function resulted in 126 patients in group A (21%), 7 in group B (1%), 414 in group C (68%) and 60 (10%) in group D. (Figure 1). Groups differed demographically in terms of: age (P<0.001), gender (P<0.001) and EuroScore II score (Table 3). Patients in groups A and C also had significantly higher maximum transvalvular gradients (P=0.019), while those in groups B and D presented with larger LV dimensions (P<0.001).

Full table
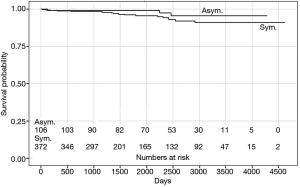
Median FU time was 5.75 years (IQR 3.24–8.00 years); FU was completed by 489 patients (81%). During FU, 25 (19%) asymptomatic patients (groups A and B) died, 3 (3%) of whom died of cardiovascular (CV) causes. In the symptomatic cohort (groups C and D), 103 patients (22%) died, 18 (4%) of whom died of CV causes. The presence of preoperative symptoms did not have a significant impact on overall mortality (P=0.085) or on CV mortality (P=0.201) (Figure 2). Patients with depressed LV function (groups B and D) displayed a higher long-term CV mortality rate (P=0.015); however, no significant difference was observed in the all-cause mortality rate (P=0.329) (Figure 3). No significant differences in all-cause (P=0.315) or CV (P=0.081) mortality was observed between the four groups.

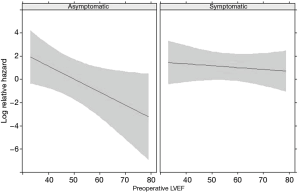
Multivariate analysis showed that preserved LVEF was a protective factor for asymptomatic patients (P=0.021), while preoperative LVEF did not affect the mortality rate in symptomatic patients (HR 0.88; 95% CI, 0.54–1.44) (Figure 4). Correspondingly, group B patients were found to be at a higher risk of long-term mortality compared to the other groups (P=0.011). Age was found to be the only other independent risk factor for long-term CV and all-cause mortality (HR 6.46; 95% CI, 2.22–18.76).
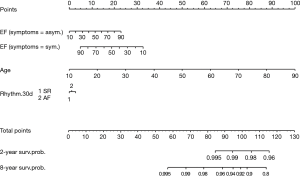
Ejection fraction, age and cardiac rhythm at discharge were combined in a nomogram to calculate a long-term CV mortality risk score (Figure 5). Every variable was weighted with points ranging from 0 to 100. The sum of each score was used to estimate the 2- and 8-year survival probability. The choice of 2 and 8 years was made based on to the I–III quartiles for the FU duration for the study population.
Discussion
According to current international guidelines, surgical treatment of severe AVS is currently given a class I recommendation only when the patient presents with reduced contractility or symptoms, including those incited by a stress test. We sought to examine the long-term outcomes of a large cohort of patients who underwent AVR for severe AVS at our institution and evaluate their indication to surgery compared to international guidelines.
The main findings of this study are: (I) the presence of preoperative symptoms did not affect long-term mortality after AVR; and (II) reduced contractility (LVEF <50%) had a significant negative impact on the survival of asymptomatic patients surgically treated for severe AVS (P=0.021). These results demonstrated that AVR in symptomatic patients treated according to current international guidelines provides good long-term survival. However, asymptomatic patients who undergo AVR with a depressed LVEF have a poor outcome in terms of mortality over long-term FU, which suggests that referring asymptomatic patients for surgery before the appearance impaired ventricular function could improve survival after AVR.
Interestingly, the presence of a concomitant AR up to moderate, was not observed as a possible associated risk factor.
Many authors have reported that delaying AVR until the onset of symptoms or the appearance of reduced LVEF is associated with worse long-term outcomes (11). For example, Kang et al. (9) recorded a 24% mortality rate at 6 years in asymptomatic patients with preserved LVEF who were medically treated. Nevertheless, they claimed that early surgery should be reserved for patients with very severe AVS. Pai et al. (12) compared patients with severe AVS who had undergone surgery with those who had not and noticed that, among the latter, 2- and 5-year mortality increased dramatically, independent of clinical, echocardiographic, and pharmacological predictors. Indeed, AVR was the only factor, which improved survival, even in asymptomatic patients. Similar results were obtained by the Mayo Clinic research group, who found that the early outcomes of AVR was comparable in symptomatic and asymptomatic patients, while not performing surgery was a risk factor for death (13). The most accredited hypothesis (14,15) regarding the observed worse outcomes for non-operated asymptomatic severe AVS patients is that delaying surgery leads to irreversible myocardial damage, leading to fibrosis and myocardial stiffness, and that this disease progression contributes to increased long-term mortality, even after AVR.
The only echocardiographic parameter that has been recognized as a risk factor for survival by several authors (7,9,16), apart from LVEF, is the degree of AVS, as measured in terms of aortic jet velocity, and its rate of progression. Rosenhek et al. (7) found that aortic jet velocity was significantly higher in patients who had cardiac events than in those who did not (P<0.001). We observed a significant difference in transvalvular gradients between patients with preserved LVEF and patients with reduced LVEF (groups B and D) (P=0.019). Given that transvalvular gradients are derived from jet velocity, groups B and D could correspond to the condition described as “low flow-low gradient aortic stenosis” (LF/LG), which is related to worse outcomes after AVR, as described in the literature. Pereira et al. (17) claimed that it is advisable to operate on patients with asymptomatic LF/LG aortic stenosis, as AVR is the only predictor of improved survival in this group (P<0.0001). Similar results were obtained by Lancellotti et al. (10), who analyzed flow patterns in asymptomatic severe AVS. They found that LF/LG is the most unfavorable flow pattern in terms of cardiac event-free survival and strongly recommended early surgery for this specific group of patients.
The current management of asymptomatic AVS patients with LVEF >50% involves close monitoring while awaiting the onset of symptoms; stress testing and careful echocardiographic monitoring of LVEF and transvalvular gradients are also recommended (18-20). Based on our results, however, it is our belief that early referral by cardiologists of every patient with severe AVS is important, before left ventricle deterioration begins. Detractors of early surgery (7,21) oppose it claiming that the risk of surgery-related death outweighs the benefit of surgery. Today, however, perioperative mortality is extremely low (1.8%) at most experienced centers (22). Moreover, adverse events related to heart valve prostheses are extremely rare (23-26). We calculated a risk score based on preoperative risk factors, ventricular function, and symptomatic status to predict long-term mortality in patients who undergo AVR. The possibility of calculating a long-term survival score enables the best surgical option for each patient to be chosen because of preoperative risk factors.
Currently, there are three ongoing trials that face the issue of early treatment of severe AVS (27-29). The first two are randomized trials: the ESTIMATE trial (Early Surgery for Patients with Asymptomatic Aortic Stenosis) and the AVATAR trial (Aortic Valve Replacement Versus Conservative Treatment in Asymptomatic Severe Aortic Stenosis), that are enrolling and randomizing patients towards conventional surgery or conservative approach. In both trials, the inclusion criteria include a low operative risk (EuroScore II <5% or STS score <8%) and the primary outcome is a combination of cardiac mortality and morbidity at a minimum of 1-year FU. The third, and more challenging study, is the EARLY TAVR trial (Evaluation of Transcatheter Aortic Valve Replacement Compared to SurveilLance for Patients with AsYmptomatic Severe Aortic Stenosis), a prospective, randomized, controlled, multi-center trial where patients with asymptomatic isolated severe AVS are randomized 1:1 to receive either a transcatheter aortic valve replacement (TAVR) with the Edwards SAPIEN 3 THV or clinical surveillance; the primary endpoint is a composite of all-cause death, all stroke, and unplanned cardiovascular hospitalization, with a time frame of 2 years. The existence of these trials gives strength to our suggestions, especially in the era of TAVR, a procedure that is comparably low risk and allows a fast recovery.
Finally, we calculated a risk score based on risk factors, ventricular function and symptomatic status, in order to predict long-term mortality in patients who undergo AVR. The possibility of calculating a long-term survival score enables the best surgical option for each patient to be chosen because of preoperative risk factors.
Limitations of this study include: (I) the small number of patients in group B; (II) the lack of long-term analysis of adverse events other than death; (III) no estimation of AVS severity by means of jet velocity and calcium score; (IV) the evaluation of LV function only by means of LVEF and not with more complete kinetic parameters, such as speckle-tracking echocardiography; and (V) the preoperative clinical evaluation, and subsequent symptomatic status determination, was performed using only NYHA functional classification.
Conclusions
The analysis of our over 10-year AVR experience confirms that current international Class I indications for symptomatic patients ensure good long-term survival. However, in our series, class I indications for asymptomatic patients with reduced LVEF are associated with poor long-term survival. According to these data, it seems that the strategy of “watchful waiting” results in lost opportunities for more optimal outcomes such as LV function preservation. Our results suggest that early surgery should be considered also for asymptomatic patients with preserved LVEF, particularly in cases of very low operative risk.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Institutional review board evaluation was waived based on the retrospective design of the study. Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
References
- Ross J Jr, Braunwald E. Aortic stenosis. Circulation 1968;38:61-7. [Crossref] [PubMed]
- Schwarz F, Baumann P, Manthey J, et al. The effect of aortic valve replacement on survival. Circulation 1982;66:1105-10. [Crossref] [PubMed]
- Horstkotte D, Loogen F. The natural history of aortic valve stenosis. Eur Heart J 1988;9:57-64. [Crossref] [PubMed]
- Kelly TA, Rothbart RM, Cooper CM, et al. Comparison of outcome of asymptomatic to symptomatic patients older than 20 years of age with valvular aortic stenosis. Am J Cardiol 1988;61:123-30. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. ESC/EACTS Guidelines for the management of valvular heart disease: The Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2017.2017. [Epub ahead of print].
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e1159-95. [Crossref] [PubMed]
- Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000;343:611-7. [Crossref] [PubMed]
- Pellikka PA, Nishimura RA, Bailey KR, et al. The natural history of adults with asymptomatic, hemodynamically significant aortic stenosis. J Am Coll Cardiol 1990;15:1012-7. [Crossref] [PubMed]
- Kang DH, Park SJ, Rim JH, et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation. 2010;121:1502-9. [Crossref] [PubMed]
- Lancellotti P, Magne J, Donal E, et al. Clinical outcome in asymptomatic severe aortic stenosis: insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol 2012;59:235-43. [Crossref] [PubMed]
- Zhao Y, Owen A, Henein M. Early valve replacement for aortic stenosis irrespective of symptoms results in better clinical survival: a meta-analysis of the current evidence. Int J Cardiol 2013;168:3560-3. [Crossref] [PubMed]
- Pai RG, Kapoor N, Bansal RC, et al. Malignant natural history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Ann Thorac Surg. 2006;82:2116-22. [Crossref] [PubMed]
- Brown ML, Pellikka PA, Schaff HV, et al. The benefits of early valve replacement in asymptomatic patients with severe aortic stenosis. J Thorac Cardiovasc Surg 2008;135:308-15. [Crossref] [PubMed]
- Pellikka PA, Sarano ME, Nishimura RA, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation. 2005;111:3290-5. [Crossref] [PubMed]
- Mihaljevic T, Nowicki ER, Rajeswaran J, et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg 2008;135:1270-8; discussion 1278-9. [Crossref] [PubMed]
- Rosenhek R, Maurer G, Baumgartner H. Should early elective surgery be performed in patients with severe but asymptomatic aortic stenosis? Eur Heart J 2002;23:1417-21. [Crossref] [PubMed]
- Pereira JJ, Lauer MS, Bashir M, et al. Survival after aortic valve replacement for severe aortic stenosis with low transvalvular gradients and severe left ventricular dysfunction. J Am Coll Cardiol 2002;39:1356-63. [Crossref] [PubMed]
- Otto CM. Valvular aortic stenosis: disease severity and timing of intervention. J Am Coll Cardiol. 2006;47:2141-51. [Crossref] [PubMed]
- Manning WJ. Asymptomatic aortic stenosis in the elderly: a clinical review. JAMA 2013;310:1490-7. [Crossref] [PubMed]
- Dal-Bianco JP, Khandheria BK, Mookadam F, et al. Management of asymptomatic severe aortic stenosis. J Am Coll Cardiol 2008;52:1279-92. [Crossref] [PubMed]
- Carabello BA. Timing of valve replacement in aortic stenosis. Moving closer to perfection. Circulation 1997;95:2241-3. [Crossref] [PubMed]
- AGENAS. Programma Nazionale Esiti. Available online: http://95.110.213.190/PNEed14/index.php
- Johnston DR, Soltesz EG, Vakil N, et al. Long term durability of bioprosthetic aortic valves: implications from 12,569 implants. Blackstone. Ann Thorac Surg 2015;99:1239-47. [Crossref] [PubMed]
- Brennan JM, Edwards FH, Zhao Y, et al. DEcIDE AVR (Developing Evidence to Inform Decisions about Effectiveness–Aortic Valve Replacement) Research Team. Long-term safety and effectiveness of mechanical versus biologic aortic valve prostheses in older patients: results from the Society of Thoracic Surgeons Adult Cardiac Surgery National Database. Circulation 2013;127:1647-55. [Crossref] [PubMed]
- Colli A, Marchetto G, Salizzoni S, et al. The TRIBECA study: (TRI)fecta (B)ioprosthesis (E)valuation versus (C)arpentier Magna-Ease in (A)ortic position. Eur J Cardiothorac Surg 2016;49:478-85. [Crossref] [PubMed]
- Colli A, Verhoye JP, Heijmen R, et al. Low-dose acetyl salicylic acid versus oral anticoagulation after bioprosthetic aortic valve replacement. Final report of the ACTION registry. Int J Cardiol 2013;168:1229-36. [Crossref] [PubMed]
- ClinicalTrial.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT02627391, Early surgery for patients with asymptomatic aortic stenosis (ESTIMATE); 2015 Dec 10 [cited 2017 Dec 11]. Available online: https://clinicaltrials.gov/ct2/show/NCT02627391
- ClinicalTrial.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT02436655, Aortic valve replacement versus conservative treatment in asymptomatic severe aortic stenosis (AVATAR); 2015 May 7 [cited 2017 Dec 11]. Available online: https://clinicaltrials.gov/ct2/show/NCT02436655
- ClinicalTrial.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT03042104, Evaluation of transcatheter aortic valve replacement compared to surveillance for patients with asymptomatic severe aortic stenosis (EARLY TAVR); 2017 Feb 3 [cited 2017 Dec 11]. Available online: https://clinicaltrials.gov/ct2/show/NCT03042104


