Management of thymic tumors: a European perspective
Introduction
Thymic tumors are infrequent tumors, although they represent the most common adult tumors in the anterior mediastinal compartment. Thymic tumors represent a wide variety of tumors, and the the most recent histologic classification clearly classifies them in thymomas, thymic carcinomas (TC) and neuroendocrine thymic tumors (NETT). Although thymic tumors have traditionally been considered as orphan diseases, in the last few decades there has been an increased interest in thymic malignancies in the scientific community, and a number of thymic tumors working group and international thymic interest groups have been instituted all over the world. In Europe, the European Society of Thoracic Surgeons (ESTS) established a thymic working group in 2010 with the intent to provide a platform among ESTS member with a specific interest in thymic malignancies.
The present review illustrates the most recent results of the ESTS thymic working group, its collaboration with other major international thymic groups, and the future projects of the group.
Thymic tumors: demographics, clinical presentation and diagnosis
Thymic tumors are rare neoplasms with an overall incidence in the USA of 0.15 cases per 100,000 person-year (1). They are the most frequent anterior mediastinal tumors in adults; they can occur in all ages, with a demonstrated peak around 30-40 years of age in thymomas with Myasthenia Gravis (MG) and 60-70 years of age in those without MG (2). Men and women are affected with the same frequency according to most series. Thymic tumors are divided into thymomas, TC and NETT. About 30% of thymoma patients are asymptomatic, while about 60-70% of patients with TC and NETT have symptoms at presentation. The most reported symptoms in any thymic tumor include local symptoms. Chest pain, cough, shortness of breath are present in both capsulated and invasive forms, while in case of invasive neoplasms, superior vena cava (SVC) syndrome, haemidiaphragm paralysis (phrenic nerve involvement) and hoarseness (recurrent laryngeal nerve infiltration) are frequently observed. Pleural effusion and chest pain are also observed in case of tumor pleural spread.
Thymic tumors are frequently associated with parathymic (paraneoplastic) diseases; Table 1 illustrates the most frequent associated diseases. MG is, by far, the most commonly associated paraneoplastic disease in thymoma patients: most series report that 30% to 50% of thymoma patients have MG, while 10-15% of MG patients present with a thymoma. Five percent of thymoma patients with MG have more than one paraneoplastic syndrome. Among non-MG paraneoplastic syndromes, Pure Red Cell Aplasia (PRCA) and Hypogammaglobulinemia (Good’s syndrome) are the most frequently observed conditions, occurring in up to 5% of the patients (3).

Full table
Thymic carcinoma is rarely associated with MG, with a variable prevalence ranging from 0% to 30% depending upon the series. Very rarely, TCs have been found to be associated with polymyositis or dermatomyositis or erithrocytosis (4,5).
NETT are frequently associated with endocrinopathies (up to 30% of the cases), including Cushing’s syndrome, multiple endocrine neoplasia type 1 (MEN-1 or Wermer syndrome, with tumors of the parathyroids, pancreatic islet cells, and pituitary gland), and growth hormone releasing hormone (GHRH) hyper-secretion with ectopic acromegaly. Other less frequent syndromes include prolactin secretion, MEN-2, peripheral neuropathy, Eaton-Lambert syndrome. Carcinoid syndrome and MG are exceptional in NETT (6).
Finally, thymoma patients have an increased (2-fold higher risk) risk to develop second malignancies.
Diagnosis of thymic tumors includes clinical examination, radiologic imaging and cyto-histologic biopsy in selected cases. Clinical examination may reveal signs and symptoms of associated parathymic syndromes or of local invasion (SVC syndrome). Computed tomography (CT) scan is considered the imaging modality of choice in the initial assessment as well as in the follow-up of patients with thymic malignancies (7-9). MRI is of little utility in diagnosis of thymic malignancies, except in case of suspected infiltration of the heart and great vessels (10). PET scan has been evaluated in its ability to differentiate thymic hyperplasia from thymoma, low-risk vs. high-risk thymomas, and thymoma vs. thymic carcinoma, although it is still less frequently used as compared to its widespread utilization in other thoracic neoplasms.
Cyto-histological diagnosis is required, although less frequently than in the past. Refinements in imaging techniques resulted in an improved diagnostic yield, and the need for a mediastinal biopsy has dramatically decreased in the recent years. There is a general agreement, however, that biopsy should be reserved in case of undefined CT findings which may suggest lymphoma, or in case of unresectable tumours before induction chemotherapy or definite chemo-radiotherapy (11).
Staging and histologic classification
No official stage classification system for thymic malignancies has been defined by the Union Internationale Contre le Cancer (UICC) and the American Joint Commission on Cancer (AJCC) and several different systems have been proposed in the past (12). The most used staging system is the Masaoka staging classification, proposed in 1981 (13), further refined with little modifications in 1994 (14) (Table 2). The classification includes four stages based upon the local extent of the disease. N factor is of limited value in the Masaoka classification. A new TNM-based system is expected in 2017, through a collaborative effort between the International Thymic Malignancies Interest Group (ITMIG) and the International Association for the Study of Lung Cancer (IASLC).

Full table
Similar to staging, a number of different histological classifications of thymic tumors have been proposed in the past 50 years, and a considerable debate has occurred among pathologists which continues until now. After a long period of controversies, in 1999 the World Health Organization (WHO) (15) reached a consensus on histologic classification of thymomas based upon both morphology and the lymphocyte to epithelial cell ratio using letters and numbers and identifying 6 subtypes: type A (medullary); type AB (mixed); type B1 (organoid); type B2 (cortical); type B3 (well-differentiated thymic carcinoma), and type C (thymic carcinoma). The classification was updated in 2004 (16) (Table 3), and the most important modification was the inclusion of type C thymomas into a separate type of thymic tumors (thymic carcinoma), further subdivided into 11 subtypes including NETT. Despite this elegant histologic differentiation into different subtypes, the prognostic significance of histology in thymic tumors, with the exception of thymic carcinoma, has never been validated in large-scale studies.
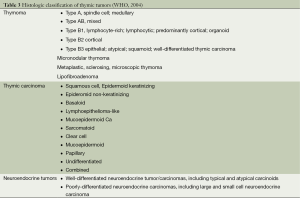
Full table
Treatment of thymic tumors
A number of papers have been published in the literature in the past decades investigating possible prognostic factors in thymic tumors. A recent review analyzed prognostic factors for survival and for recurrence in thymic tumors exploring the most recent literature (17). The only validated prognostic factors for both survival and recurrence were the stage at presentation (Masaoka or Masaoka/Koga) and the completeness of resection. Gender and MG are consistently reported NOT to be significant predictors for either survival or recurrence. Histology according to WHO classification does not seem to be a validated prognostic factor, with the exception of thymic carcinoma. Other prognostic factors, including age, tumor size, other parathymic syndromes were inconsistently reported as significant prognostic factors.
Surgery
Surgical resection is the mainstay of treatment of thymoma, with a reported operative mortality of 2% and a complication rate of approximately 20% (18). Complete resection should be the primary goal and results depend on the localization and the size of the tumor. The ITMIG recommends en bloc resection including complete thymectomy and resection of the surrounding mediastinal fat because of the possibility of subtle macroscopically invisible invasion of the tumor. The 10-year survival rates are 90%, 70%, 55%, and 35% for stages I, II, III, and IVa thymoma, respectively The recurrence rate is 3%, 11%, 30% and 43% in resected stage I, II, III, and IVa thymoma, respectively. The disease free survival at 10 years is 94%, 88%, 56%, and 33% for stages I, II, III, and IVa, respectively (19).
Most centers recommend a sternotomy as the optimal incision for thymoma as it might not be possible to perform a complete thymectomy via thoracotomy (20). Transcervical approach has also been used for this purpose. Minimally invasive approaches including video-assisted thoracoscopic surgery (VATS) and robotic-assisted thoracoscopic surgery (RATS) for early stage thymomas have been reported (21-23) and are gaining popularity in specialized centers. Especially RATS allows an excellent exposure and precision of resection in tumors of adequate size and location.
Radiotherapy (RT)
Thymic tumors have a tendency to local recurrence, and show moderate-high radiosensitivity profiles. This has always been considered a prerequisite for the adoption of RT in the whole treatment strategy. RT may be delivered before surgery, after surgery, and as exclusive treatment in patients not eligible to surgical intervention or for treatment of recurrent tumors. The delivered doses varies according to the clinical setting, ranging from 45 Gy as preoperative therapy, to 45-55 Gy as postoperative, to 60-66 Gy as exclusive treatment, with conventional fractionation (1.8-2.0 Gy/day) (24).
Postoperative RT is usually performed within 3 months from the surgical procedure, for a total dose of 50-54 Gy in 1.8-2.0 Gy fractions. In Masaoka Stage I, adjuvant RT has no role. In stage II, the largest series so far found either no differences with or without RT (25), or even a detrimental effect (26); In stage III, adjuvant RT has a well-established role among clinicians, although the evidence so far is low. The most recent trials failed to show any significant advantage (25,27,28).
Radical “curative” RT is customarily adopted in inoperable patients, or in patients deemed inoperable after induction chemotherapy. Combined chemo-radiation is usually delivered in a sequential manner to a total dose of 54-70 Gy. The response rate reaches 70%, with a 5-year survival projection of 70-80%: these results are similar to those reported after surgery with incomplete resection (24,29).
Chemotherapy
Thymic tumors are generally chemosensitive, and thymomas are more sensitive to chemotherapy than TC. Chemotherapy strategies comprise chemotherapy used as initial treatment and as treatment in case of recurrence. Chemotherapy as initial treatment can be further divided in chemotherapy with curative intent (primary/preoperative/induction chemotherapy or postoperative chemotherapy) and chemotherapy with palliative intent. Exclusive (palliative) chemotherapy is administrated in patients medically or technically not suitable for surgical procedures or in patients with metastatic disease (30).
The major goal of primary chemotherapy is to downstage the tumor prior to surgery. A recent Cochrane analysis was performed (31) to evaluate the role of induction therapy in thymic tumors: 49 relevant randomized studies were identified; however none of them met the criteria allowed for a Cochrane analysis. Therefore all published guidelines to multi-modal treatment of thymic neoplasm remain expert opinions. On average, in patients with stage III-IV thymic neoplasms, primary chemotherapy achieves a response rate of 71% (29-100%) and surgery results in a complete resection in 68% (22-86%) (2). All used regimens are poly-chemotherapies.
In the majority of the cases post-operative therapy after resection of thymic tumors consists of RT or radio/chemotherapy. In contrast to lung cancer, since improved local control is the main focus of adjuvant therapy in thymic neoplasms, chemotherapy alone is rarely used in an adjuvant setting.
Finally, in case of metastatic spread or contraindications to local treatment with surgery or RT, exclusive chemotherapy with palliative intent is offered with reasonable results. In emergency situations like SVC syndrome or evident clinical symptoms, poly-chemotherapy is an option with a proven efficacy on QoL.
Combined radio/chemotherapy
The rationale to combine chemotherapy and radiation therapy is to augment cytotoxicity against remaining tumor cells by an additive effect of chemotherapy and radiation. Combined radio/chemotherapy can be used in the post-operative setting if a large unresectable tumor volume (R2) remains in the thorax. Furthermore radio-/chemotherapy is the definitive treatment for patients medically unfit or with a thymic neoplasm technically not resectable (32).
Targeted therapies
A recent interest has developed in the molecular and genomic analysis of thymic tumors for the evaluation of possible targeted therapies (33).
KIT is overexpressed in 2% of thymomas and in 79% of TC when analyzed by immunohistochemistry (34). There are activating mutations in exon 9, 11, 13 and 17. Imatinib is a small molecule multi-kinase inhibitor blocking KIT, Bcr-Abl, and PDGFR. However, the treatment with imatinib resulted only in short disease stabilization in phase II studies (35).
EGFR is overexpressed in 70% of thymomas and 53% of TC. Erlotinib and gefitinib are small molecule tyrosine kinase EFGR inhibitors. Unfortunately, no clear association has been described so far between specific mutations in the EGFR and activity of specific tyrosine kinase inhibitors.
Markers of angiogenesis have been investigated in thymic neoplasms. VEGF-A serum levels were elevated in serum samples of patients with TC, and VEGF-R1 and R2 were expressed in the malignant thymic tissue. Although a theoretical application of anti-angiogenetic agents (sunitinib, sorafenib and antibodies binding VEGF-bevacizumab and aflibercept) is appealing, the published data indicate only a weak clinical efficacy (36,37).
Thymic tumors: the European contribution by ESTS
By definition, a rare and orphan disease is one that affects fewer than 200,000 individuals in the United States. Thymic tumors are classified as orphan diseases, due to their low prevalence. As a consequence, most of our knowledge on these tumors has been based so far from single-institution case series, usually spanned over a long time period to collect a sufficient number of cases to draw a statistically appropriate analysis. For this reason, advancements in management strategies have been slow so far. In addition, at least until recently, the lack of coordination among centres with sufficient experience resulted a major obstacle.
A dramatic improvement occurred in the past decade, when the most important thoracic societies addressed this issue by establishing dedicated thymic groups into their structure.
A major step forward in the scientific advancement of thymic tumors has been the creation, in 2010, of the ITMIG (www.itmig.org) (38), which was endorsed and supported by the most representatives medical and surgical societies around the globe. According to its constitution, ITMIG’s mission is to promote the advancement of clinical and basic science related to thymic malignancies. It provides infrastructure for international cooperation, it maintains close collaboration with other related organizations and it facilitates the spread of knowledge about thymic neoplasms. ITMIG works in close cooperation with the most important thymic groups of the international medical societies including the ESTS, the European Association of Cardio-thoracic Surgeons (EACTS), the Japanese Association for Research of the Thymus (JART). It is a multidisciplinary organization, involving thoracic surgeons, radiation and medical oncologists, pathologists, pulmonologists, radiologists and basic science researchers. It includes more than 600 members from all continents.
Europe has a long-standing tradition in the research of thymic tumors, and many European Institutions from France and Italy have been leading centres over the past 30 years. A substantial number of papers have been published from European centres, which have constituted landmark manuscripts for the scientific community. Not surprisingly, therefore, the ESTS, which is the most representative general thoracic surgical society in Europe, started its thymic working group in 2010 with the intent to provide a common platform to its members interested in thymic tumors.
The thymic group first met at the ESTS annual meeting in Valladolid, Spain in 2010 where a list of 35 interested centres were identified and a survey was designed about the current management of thymic tumors among ESTS members. The results of the survey were published in 2011 (39).
At the next ESTS annual meeting in Marseille, France in 2011 the thymic group launched the ESTS thymic retrospective database project to collect data of patients submitted to surgical resection of thymic tumors among any interested ESTS centre. The dataset was designed in collaboration with ITMIG to have similar datafields for future common projects.
At the 2012 ESTS annual meeting in Essen, Germany, the preliminary results of the thymic retrospective database were presented, and a dedicated thymic section in the ESTS Registry was funded and provided using the official platform of the ESTS database.
Finally, at the 2013 ESTS annual meeting in Birmingham, UK, the ESTS prospective thymic database was officially launched into the thymic section of the ESTS Registry, where any ESTS member may upload his/her patients with thymic tumors prospectively.
As mentioned above, therefore, ESTS represents the leading European force in the study of thymic tumors, with an unprecedented collaborative effort among the participating institutions and an enthusiasm which will certainly stimulate new projects in the future.
The major products of this extraordinary collaborative effort have been three papers which have been published in the last three years, covering important aspects of the management of thymic tumors.
The ESTS survey on management of thymic tumors (39)
After its establishment in 2010, the first move of the ESTS thymic working group was to test the differences of management of thymic tumors in the ESTS community. Due to their rarity, there are no current guidelines or consensus statements about appropriate management of thymic tumors, and most of the current practice is based upon personal series and expertise of the individual centres. For this reason, a survey was deemed a useful tool to investigate the state-of-the-art of the current practice.
A questionnaire was designed, which was circulated by email to all ESTS members. The questionnaire included 25 points organized in seven sections:
- Volume of activity of the participating center;
- Preoperative assessment;
- Histology and staging system employed;
- Details on surgical resection;
- Pre- and post-operative treatments for invasive thymic tumors;
- Management of recurrence, thymic carcinoma and Stage IVa thymic tumors;
- The organization and logistics of the thymic activity of the centre.
Overall, 44 centres replied and their answers were used for the results. Although ESTS is primarily a European thoracic society, it is open to any centre from any country all over the world who has interest in general thoracic surgery. Out of the 44 responders, 33 were from Europe, 7 from USA/Canada/South America, 4 from Asia.
The results of the study showed that there are some areas of agreement about different aspects in the diagnosis and management of thymic tumors, although there still are many “grey” areas of controversy which need to be elucidated by large-scale collaborative studies.
In particular, the study found that there is a substantial agreement on the following issues: (I) the pivotal role of CT scan in the preoperative diagnosis of thymic tumors. Last generation CT scans allow a well-definite morphological imaging of the thymic tumor, the possible invasion of the surroundings organs, and a precise differential diagnosis with other anterior mediastinal tumors, thus avoiding routine cyto-histologic confirmation; (II) as a consequence of the former, routine cyto-histologic diagnosis before surgical resection is not performed by the vast majority of the interviewed centres, unless differential diagnosis remains difficult or induction therapy is required; (III) the Masaoka staging system (although with a still considerable confusion with the updated Masaoka-Koga system) and the 2004 WHO histologic classification are used by the vast majority of the centres; (IV) the importance of a complete resection for good long-term results. Most centres correctly indicated complete resection as a major prognostic factor, although some concern still exists by some centres about resection of great vessels (innominate vein, SVC) and reconstruction, as well as the resection of the phrenic nerve in MG patients. The message of the survey was that resection/reconstruction of the great vessels is indicated for the venous conduits (innominate vein, SVC), and unilateral resection of the phrenic nerve can be accomplished, even in MG patients, while bilateral phrenic nerve resection and resection of the arterial conduits are to be avoided in most cases; (V) the surgical approach to recurrence. Most centres also indicated that a proper selection of patients candidates to surgical resection of the recurrence is mandatory, although recurrence resection is indicated whenever possible; (VI) the importance to have a dedicated pathologist for thymic tumors, along with a dedicated thymic tumor board including dedicated medical and radiation oncologists.
On the other hand, the survey clearly indicated that there is still a considerable debate about the following issues: (I) the role of PET scan for the preoperative assessment. Only half of the interviewed centres declared to routinely use PET scan, although most institutions use it on a selecetive basis with the following indications: (i) to detect a potentially invasive thymoma; (ii) in case of suspected pleural dissemination (Stage IVa); and (iii) for the assessment of response after induction therapy. The results of the survey seem to confirm some studies recently published on this topic (40,41); (II) the administration of postoperative therapies after resection of invasive (Stage II-III) thymomas. This is possibly the most controversial issue in the management of thymic tumors, which has been addressed also in the other two papers based on the ESTS retrospective database. In the survey, 60% of the interviewed centres indicated that they use postoperative RT even in Stage II thymoma, although on a selective basis (high-risk WHO histology-B2/B3; high-risk free resection margins). As for Stage III thymomas, the survey confirmed the standard practice to administer postoperative RT after resection (60%), either complete or not. Postoperative chemotherapy alone is seldom performed (7%), although postoperative radio-chemotherapy was indicated by some centers. Induction therapy is, on the other hand, a consolidated treatment modality in the scientific community, and 75% of the centres stated that they use it in case of perceived unresectable tumors; (III) treatment modality for thymic carcinoma. The general feeling from the centres was that thymic carcinoma should be treated in a multidisciplinary setting, and this prompted the ESTS thymic group to analysed thymic carcinoma separately in the ESTS retrospective database; (IV) the role of extended surgery in the treatment of thymoma extending to the pleura (Stage IVa). Although 40% of the centres replied that they may consider extrapleural pneumonectomy in these patients, the majority of the institutions seems reluctant to embark in this extensive surgery in this subset of patients.
Finally, the study addressed the question whether high-volume (>10 thymic resections/year, N=18) institutions have different diagnostic and treatment management as compared to low-volume (<10 thymic resections/year, N=26) centres. Interestingly, the approach to thymic tumors was quite similar in the two groups, indicating that a high-quality standard of management can be obtained irrespective of the number of performed resections.
Despite the limitations of the study, which are common to any survey, the major value of the ESTS survey was to provide a large, multiinstitutional, clear overview of the clinical practice in the management of thymic tumors, ranging from top-quality excellence centres to regional community hospitals all interested in the treatment of these rare tumors.
Overall results of the ESTS thymic retrospective database (42)
The ESTS thymic retrospective database was launched in 2011 and included data on patients submitted to surgery for thymic tumors from 1990 to 2010. The preliminary results were shown in 2012 and the final paper was published in 2014. The manuscript investigated possible prognostic factors for all thymic tumors on a cohort of 2,151 patients from 35 institutions. Overall survival (OS) and disease-free survival (DFS) were used as outcome measures, along with the cumulative incidence of recurrence (CIR).
The analysis was conducted examining the following points:
- Analysis of predictors of incomplete resection;
- Analysis of predictors of OS and DFS;
- Analysis of predictors of recurrence;
- Subgroup analysis on the role of adjuvant (postoperative) therapy.
Analysis of predictors of incomplete resection indicated that the probability to perform an incomplete resection increased in male patients (vs. female), in absence of MG, in larger tumors, and in high-risk thymomas (B2/B3), thymic carcinoma and NETT.
Predictors of OS and DFS were similar. Mortality increased with age, with Masaoka stage, and in incomplete resection. The ESTS study did not indicate WHO histology as a significant prognostic factor.
Predictors of recurrence were investigated after complete resection. CIR was 5%, 8% and 12% at 3, 5 and 10 years respectively. The probability of a recurrence increased with non-MG status, in larger tumors and in advanced stages.
Finally, the subgroup analysis on the role of adjuvant therapy following a complete resection of the thymic tumor indicated (using a Cox model adjusted for propensity score) that an overall beneficial effect of adjuvant therapy on OS occurred in the cohort, without major differences in other subgroups.
The ESTS cohort study is the largest collaborative retrospective study on thymic tumors published so far, and this certainly represents a major value. As any other multi-institutional retrospective study it suffers from an inhomogeneous geographical distribution, different volume activity of the participating centres, the lack of a central review of the pathology reports, etc. Nonetheless, while awaiting for the results from prospective studies, it still represents an important reference in the field of thymic tumors.
Results of the ESTS thymic retrospective database on the patients with thymic carcinoma
The third contribution of the ESTS thymic group is the subgroup analysis of patients with thymic carcinoma from the ESTS thymic retrospective database, which was presented at the ASCO meeting in June 2013 in Chicago, and which is in press in JTO. The patient population included 229 TC out of 2,265 patients with thymic tumors from 36 institutions. As in the study on the overall population, primary endpoints were OS, DFS and CIR.
Survival analysis was performed according with different covariates, and analysis of predictors for OS, DFS and recurrence was undertaken.
As for the ESTS cohort study of prognostic factors on the overall population of thymic tumors, this study represents so far the largest series of patients with thymic carcinoma submitted to surgical resection ever published.
Conclusions
We can therefore conclude that a dramatic improvement in our knowledge on the diagnosis and management of thymic tumors has occurred in the last few years (43,44). Europe is a leading force in the development of the collaborative international effort which has taken place in the most recent year. The ESTS thymic group played a major role in this effort. ESTS is actively working with the Japanese Association for Research in Thymus (JART), ITMIG and IASLC in the development of the next 8th edition of the TNM of thoracic malignancies, expected in 2017, where a new TNM-based staging system for thymic tumors will be proposed, based upon the extraordinary data collection from the three most important datasets ever collected (ITMIG, JART and ESTS). Further projects are under way in the ESTS thymic group, including the prospective collection of thymic data using the ESTS Registry, and the exploration for a possible tissue bank of thymic tumors for genomic and molecular analysis with possible therapeutic implications. All these projects can be pursued only with the dedicated enthusiasm of the people involved in the ESTS thymic group, along with all the representatives of the institutions from Europe, USA/Canada, and Asia who sent their data for the patient collection, and who actively participate in the activity of the group.
The results of this amazing European-based international cooperation led by ESTS will soon be available, resulting in a major improvement in the outcome of a patient population which until now has suffered from the difficulties common to many orphan diseases.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Engels EA, Pfeiffer RM. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer 2003;105:546-51. [PubMed]
- Venuta F, Anile M, Diso D, et al. Thymoma and thymic carcinoma. Eur J Cardiothorac Surg 2010;37:13-25. [PubMed]
- Chen J, Yang Y, Zhu D, et al. Thymoma with pure red cell aplasia and Good’s syndrome. Ann Thorac Surg 2011;91:1620-2. [PubMed]
- Inoue Y, True LD, Martins RG. Thymic carcinoma associated with paraneoplastic polymyositis. J Clin Oncol 2009;27:e33-4. [PubMed]
- Azuma Y, Shiga K, Ishii R, et al. Polymyositis with atypical pathological features associated with thymic carcinoma. Intern Med 2009;48:163-8. [PubMed]
- Benveniste MF, Rosado-de-Christenson ML, Sabloff BS, et al. Radiographics 2011;31:1847-61; discussion 1861-3.
- Ruffini E, Oliaro A, Novero D, et al. Neuroendocrine tumors of the thymus. Thorac Surg Clin 2011;21:13-23. [PubMed]
- Marom EM. Imaging thymoma. J Thorac Oncol 2010;5:S296-303. [PubMed]
- Marom EM. Advances in thymoma imaging. J Thorac Imaging 2013;28:69-80. [PubMed]
- Sadohara J, Fujimoto K, Müller NL, et al. Thymic epithelial tumors: comparison of CT and MR imaging findings of low-risk thymomas, high-risk thymomas, and thymic carcinomas. Eur J Radiol 2006;60:70-9. [PubMed]
- Detterbeck FC, Parsons AM. Thymic tumors. Ann Thorac Surg 2004;77:1860-9. [PubMed]
- Filosso PL, Ruffini E, Lausi PO, et al. Historical perspectives: The evolution of the thymic epithelial tumors staging system. Lung Cancer 2014;83:126-32. [PubMed]
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [PubMed]
- Koga K, Matsuno Y, Noguchi M, et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int 1994;44:359-67. [PubMed]
- Rosai J, Sobin L. Histological typing of tumours of the thymus. In: Rosai J, Sobin L. eds. World Health Organization, International classification of tumours. Berlin: Springer, 1999:9-14.
- Travis WD, Brambilla E, Muller-Hermelink HK, et al. Pathology and genetics of tumours of the lung, pleura, thymus and heart. In: Kleihues P, Sobin LH. eds. WHO Classification of tumours. 2nd Edition Lyon: IARC Press, 2004:145-97.
- Detterbeck F, Youssef S, Ruffini E, et al. A review of prognostic factors in thymic malignancies. J Thorac Oncol 2011;6:S1698-704. [PubMed]
- Detterbeck FC, Parsons AM. Management of stage I and II thymoma. Thorac Surg Clin 2011;21:59-67. [PubMed]
- Koppitz H, Rockstroh JK, Schüller H, et al. State-of-the-art classification and multimodality treatment of malignant thymoma. Cancer Treat Rev 2012;38:540-8. [PubMed]
- Kaiser LR. Surgical treatment of thymic epithelial neoplasms. Hematol Oncol Clin North Am 2008;22:475-88. [PubMed]
- Toker A, Sonett J, Zielinski M, et al. Standard terms, definitions, and policies for minimally invasive resection of thymoma. J Thorac Oncol 2011;6:S1739-42. [PubMed]
- Marulli G, Rea F, Melfi F, et al. Robot-aided thoracoscopic thymectomy for early-stage thymoma: a multicenter European study. J Thorac Cardiovasc Surg 2012;144:1125-30. [PubMed]
- Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [PubMed]
- Girard N, Mornex F. The role of radiotherapy in the management of thymic tumors. Thorac Surg Clin 2011;21:99-105. [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. [PubMed]
- Ruffini E, Mancuso M, Oliaro A, et al. Recurrence of thymoma: analysis of clinicopathologic features, treatment, and outcome. J Thorac Cardiovasc Surg 1997;113:55-63. [PubMed]
- Forquer JA, Rong N, Fakiris AJ, et al. Postoperative radiotherapy after surgical resection of thymoma: differing roles in localized and regional disease. Int J Radiat Oncol Biol Phys 2010;76:440-5. [PubMed]
- Korst RJ, Kansler AL, Christos PJ, et al. Adjuvant radiotherapy for thymic epithelial tumors: a systematic review and meta-analysis. Ann Thorac Surg 2009;87:1641-7. [PubMed]
- Loehrer PJ Sr, Chen M, Kim K, et al. Cisplatin, doxorubicin, and cyclophosphamide plus thoracic radiation therapy for limited-stage unresectable thymoma: an intergroup trial. J Clin Oncol 1997;15:3093-9. [PubMed]
- Girard N, Lal R, Wakelee H, et al. Chemotherapy definitions and policies for thymic malignancies. J Thorac Oncol 2011;6:S1749-55. [PubMed]
- Wei ML, Kang D, Gu L, et al. Chemotherapy for thymic carcinoma and advanced thymoma in adults. Cochrane Database Syst Rev 2013;8:CD008588. [PubMed]
- Girard N. Thymic epithelial tumours: from basic principles to individualised treatment strategies. Eur Respir Rev 2013;22:75-87. [PubMed]
- Girard N. Targeted therapies for thymic malignancies. Thorac Surg Clin 2011;21:115-23. [PubMed]
- Lamarca A, Moreno V, Feliu J. Thymoma and thymic carcinoma in the target therapies era. Cancer Treat Rev 2013;39:413-20. [PubMed]
- Giaccone G, Rajan A, Ruijter R, et al. Imatinib mesylate in patients with WHO B3 thymomas and thymic carcinomas. J Thorac Oncol 2009;4:1270-3. [PubMed]
- Cimpean AM, Raica M, Encica S, et al. Immunohistochemical expression of vascular endothelial growth factor A (VEGF), and its receptors (VEGFR1, 2) in normal and pathologic conditions of the human thymus. Ann Anat 2008;190:238-45. [PubMed]
- Ströbel P, Bargou R, Wolff A, et al. Sunitinib in metastatic thymic carcinomas: laboratory findings and initial clinical experience. Br J Cancer 2010;103:196-200. [PubMed]
- Detterbeck F. International thymic malignancies interest group: a way forward. J Thorac Oncol 2010;5:S365-70. [PubMed]
- Ruffini E, Van Raemdonck D, Detterbeck F, et al. Management of thymic tumors: a survey of current practice among members of the European Society of Thoracic Surgeons. J Thorac Oncol 2011;6:614-23. [PubMed]
- Endo M, Nakagawa K, Ohde Y, et al. Utility of 18FDG-PET for differentiating the grade of malignancy in thymic epithelial tumors. Lung Cancer 2008;61:350-5. [PubMed]
- Inoue A, Tomiyama N, Tatsumi M, et al. (18)F-FDG PET for the evaluation of thymic epithelial tumors: Correlation with the World Health Organization classification in addition to dual-time-point imaging. Eur J Nucl Med Mol Imaging 2009;36:1219-25. [PubMed]
- Ruffini E, Detterbeck F, Van Raemdonck D, et al. Tumours of the thymus: a cohort study of prognostic factors from the European Society of Thoracic Surgeons database. Eur J Cardiothorac Surg 2014. [Epub ahead of print]. [PubMed]
- Detterbeck FC, Zeeshan A. Thymoma: current diagnosis and treatment. Chin Med J (Engl) 2013;126:2186-91. [PubMed]
- Tomaszek S, Wigle DA, Keshavjee S, et al. Thymomas: review of current clinical practice. Ann Thorac Surg 2009;87:1973-80. [PubMed]


