
The false illusion of coronary thrombus device-management
Primary percutaneous coronary intervention (PCI) in ST-segment elevation myocardial infarction (STEMI) has contributed, in association with optimized antithrombotic therapy, to a dramatic decline in mortality over the last decades (1). Among the numerous mechanisms that may participate to non-optimal myocardial reperfusion during or after the ischemia-reperfusion process, distal embolization of thrombus or debris during primary PCI appears as a cause to be prevented (2,3). Several device-based strategies have been evaluated aiming at improving reperfusion success by reducing distal vessel occlusion and microvascular obstruction (4).
Filter devices for distal protection were intuitively relevant but, although visible debris could be captured, randomized studies failed to show a benefit in terms of reperfusion, final infarct size, and clinical outcome (5-7). To avoid distal embolization by mobilization of coronary thrombus during the acute phase of MI, another approach suggested to delay stenting when TIMI flow was preserved on the initial coronary angiogram. Of four randomized trials, only one (8), with the smallest population size, suggested a reduction of no-reflow and an improved myocardial salvage while the three others (9-11) reported no benefit on microvascular obstruction and clinical endpoints.
In addition to these approaches, thrombus-aspiration (TA) devices have been developed to remove the coronary thrombus from the culprit vessel rather than squeeze or dislodge it during stenting. However, the potential improvements of TA on the microcirculation do not consistently translate into clinical benefits. Indeed, a beneficial effect of TA was observed in the single-center TAPAS trial (12) but there was no significant survival benefit of TA in the TASTE trial (13) and in the TOTAL trial (14), the largest randomized trial in this setting with more than 10,700 STEMI patients. In an analysis of the pooled individual data of the 18,306 patients included in TAPAS, TASTE, and TOTAL, a numerically lower risk of cardiovascular death at 30 days (2.4% vs. 2.9%) was reported with TA, at the expense of a numerically higher risk of stroke or transient ischemic attack (0.8% vs. 0.5%), but none of these differences reached statistical significance (P=0.06 for both comparisons) (15). Following these data, routine TA in primary PCI patients was downgraded to a class III recommendation (16). However, the outstanding question remains whether a selective use of TA could improve microvascular reperfusion and achieve a substantial benefit in the subset of patients with a high risk of distal embolization (patients with a poor initial TIMI flow or with a large thrombus burden) (17). Although no large randomized study was performed on this specific situation, a recent sub-analysis of the TOTAL trial partly answers the question (18). In patients with high thrombus burden (TIMI classification thrombus grade ≥3, including total artery occlusion), that finally concerned the majority of patients (89% of the population), TA did not reduce the composite endpoint of cardiovascular death, MI, heart failure or cardiogenic shock and was associated with an increased risk of stroke within 48 h of the procedure. This analysis emphasizes the need for an adequate procedural technique when aspiration is performed despite the negative trials: deep seating of the guiding catheter, slow advancement and retrieval of the catheter with continuous aspiration. However, thrombus grade after wire crossing, a key factor for decision-making, was not documented and distal embolization was significantly reduced following TA.
Finally, direct stenting (DS), without balloon predilation, is another strategy to reduce vessel wall damage and the risk of thrombus fragmentation and embolization (19). On the other hand, there are significant downsides to DS including: underestimation of the true vessel size, failure to cross in tortuous or calcified lesions, incomplete lesion coverage, inadequate stent expansion and late stent malapposition, that may increase the risk of restenosis or stent thrombosis. A recent meta-analysis of seven trials of DS versus conventional stenting concerning 10,900 patients treated by drug eluting stents showed that DS was associated with reduction of soft endpoints (procedural time, radiation and contrast exposure) and, more interestingly, death/myocardial infarction and target lesion revascularization (20). However, only three small randomized trials evaluating DS have been conducted in STEMI, with conflicting results. While two studies found a benefit on the post-procedure TIMI 3 flow rate (21) or on ST-segment resolution and a composite endpoint of no- or slow-reflow, electrocardiographic changes, and clinical outcome (22), the most recent and largest study (23) found no difference in surrogate endpoints and 5-year clinical outcome.
Acknowledging these controversial results, the different approaches could also achieve synergy to reduce distal embolization. TA has the potential to remove the thrombus from the culprit lesion allowing a better evaluation of lesion length and reference vessel diameter that could facilitate and optimize DS. The first randomized trial evaluating this association (24) included a relatively small population of 196 STEMI patients and failed to demonstrate better post-procedural ST-segment resolution after TA associated to DS in comparison to conventional stenting after predilation.
Along this line, Mahmoud et al., performed the largest study comparing DS with conventional stenting and the interaction with TA in STEMI patients from a pooled analysis of the randomized TAPAS, TASTE and TOTAL trials (25). One third of the 17,329 patients underwent DS. DS was performed significantly more often in patients assigned by randomization to TA than in those assigned to conventional PCI (41% vs. 22%; P<0.001), suggesting that TA does indeed facilitate DS. A propensity matching was performed to reduce bias related to the non-randomized study design for DS, resulting in a cohort of 10,944 patients. There was no evidence of benefit or harm of DS and no significant interaction with TA, suggesting lack of synergy of the two procedures. Particularly, there was no significant difference for 1-year cardiovascular death or cerebrovascular events between DS and conventional stenting (2.7% vs. 3.0%, adjusted P=0.55 and 1.2% vs. 0.9%, adjusted P=0.98, respectively). This analysis failed to demonstrate a favorable effect of DS on myocardial perfusion which had been the putative mechanism for a possible clinical benefit. Indeed, considering the patients with available data on myocardial reperfusion, there was only a trend to improved ST-segment resolution by DS (P=0.06) but no significant difference for myocardial Blush grade after adjustment. Even if this study, based on a very large population, addresses an important issue, some limitations should be noted: the post-hoc nature of the analysis, the different periods of the three studies, randomization for TA but DS performed selectively at operator’s discretion, myocardial perfusion data available only for two of the three trials, different degrees of operator’s experience, no specific evaluation or adjustment of anti-thrombotic therapy. The main limitation, inherent to the conduct of randomized studies, is the evaluation of a systematic use of TA or DS when clinical practice allows individualized approach based on clinical presentation, procedural considerations and operator’s experience.
The different device-based strategies evaluated to reduce distal embolization and improve myocardial reperfusion, are illustrated in Figure 1, bearing in mind that these approaches are complementary with anti-thrombotic therapy including GPIIb/IIIa inhibitors and potent P2Y12 inhibitors.
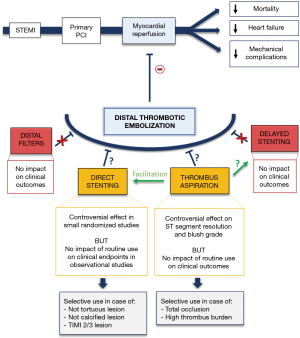
In conclusion, device-based strategies may have a limited effect to reduce microvascular obstruction and distal embolization in STEMI management. However, even if no meaningful benefit on clinical outcomes was detected, DS can still be performed as no safety concerns were raised.
Acknowledgements
None.
Footnote
Conflicts of Interest: B Lattuca has received research grants from Biotronik, Daiichi-Sankyo, Fédération Française de Cardiologie, Institute of Cardiometabolism and Nutrition; consultant fees from Daiichi-Sankyo and Eli Lilly; and lecture fees from AstraZeneca and Novartis. G Montalescot has received research grants from Abbott, Amgen, Actelion, AstraZeneca, Bayer, Boehringer Ingelheim, Boston-Scientific, Bristol-Myers Squibb, Beth Israel Deaconess Medical, Brigham Women’s Hospital, Cardiovascular Research Foundation, Daiichi-Sankyo, Idorsia, Lilly, Europa, Elsevier, Fédération Française de Cardiologie, ICAN, Medtronic, Journal of the American College of Cardiology, Lead-Up, Menarini, MSD, Novo-Nordisk, Pfizer, Sanofi, Servier, The Mount Sinai School, TIMI Study Group, WebMD.
References
- Puymirat E, Simon T, Cayla G, et al. Acute Myocardial Infarction: Changes in Patient Characteristics, Management, and 6-Month Outcomes Over a Period of 20 Years in the FAST-MI Program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation 2017;136:1908-19. [Crossref] [PubMed]
- Heusch G. The Coronary Circulation as a Target of Cardioprotection. Circ Res 2016;118:1643-58. [Crossref] [PubMed]
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007;357:1121-35. [Crossref] [PubMed]
- Poli A, Fetiveau R, Vandoni P, et al. Integrated analysis of myocardial blush and ST-segment elevation recovery after successful primary angioplasty: Real-time grading of microvascular reperfusion and prediction of early and late recovery of left ventricular function. Circulation 2002;106:313-8. [Crossref] [PubMed]
- Kelbaek H, Terkelsen CJ, Helqvist S, et al. Randomized comparison of distal protection versus conventional treatment in primary percutaneous coronary intervention: the drug elution and distal protection in ST-elevation myocardial infarction (DEDICATION) trial. J Am Coll Cardiol 2008;51:899-905. [Crossref] [PubMed]
- Stone GW, Webb J, Cox DA, et al. Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: a randomized controlled trial. JAMA 2005;293:1063-72. [Crossref] [PubMed]
- Gick M, Jander N, Bestehorn HP, et al. Randomized evaluation of the effects of filter-based distal protection on myocardial perfusion and infarct size after primary percutaneous catheter intervention in myocardial infarction with and without ST-segment elevation. Circulation 2005;112:1462-9. [Crossref] [PubMed]
- Carrick D, Oldroyd KG, McEntegart M, et al. A randomized trial of deferred stenting versus immediate stenting to prevent no- or slow-reflow in acute ST-segment elevation myocardial infarction (DEFER-STEMI). J Am Coll Cardiol 2014;63:2088-98. [Crossref] [PubMed]
- Kim JS, Lee HJ, Woong Yu C, et al. INNOVATION Study (Impact of Immediate Stent Implantation Versus Deferred Stent Implantation on Infarct Size and Microvascular Perfusion in Patients With ST-Segment-Elevation Myocardial Infarction). Circ Cardiovasc Interv 2016.9. [PubMed]
- Belle L, Motreff P, Mangin L, et al. Comparison of Immediate With Delayed Stenting Using the Minimalist Immediate Mechanical Intervention Approach in Acute ST-Segment-Elevation Myocardial Infarction: The MIMI Study. Circ Cardiovasc Interv 2016;9:e003388. [Crossref] [PubMed]
- Kelbæk H, Høfsten DE, Køber L, et al. Deferred versus conventional stent implantation in patients with ST-segment elevation myocardial infarction (DANAMI 3-DEFER): an open-label, randomised controlled trial. Lancet 2016;387:2199-206. [Crossref] [PubMed]
- Svilaas T, Vlaar PJ, van der Horst IC, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med 2008;358:557-67. [Crossref] [PubMed]
- Fröbert O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med 2013;369:1587-97. [Crossref] [PubMed]
- Jolly SS, Cairns JA, Yusuf S, et al. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med 2015;372:1389-98. [Crossref] [PubMed]
- Jolly SS, James S, Džavík V, et al. Thrombus Aspiration in ST-Segment-Elevation Myocardial Infarction: An Individual Patient Meta-Analysis: Thrombectomy Trialists Collaboration. Circulation 2017;135:143-52. [Crossref] [PubMed]
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119-77. [Crossref] [PubMed]
- De Luca G, Ernst N, Zijlstra F, et al. Preprocedural TIMI flow and mortality in patients with acute myocardial infarction treated by primary angioplasty. J Am Coll Cardiol 2004;43:1363-7. [Crossref] [PubMed]
- Yap SC, Harris L, Silversides CK, et al. Outcome of intra-atrial re-entrant tachycardia catheter ablation in adults with congenital heart disease: negative impact of age and complex atrial surgery. J Am Coll Cardiol 2010;56:1589-96. [Crossref] [PubMed]
- Barbato E, Marco J, Wijns W. Direct stenting. Eur Heart J 2003;24:394-403. [Crossref] [PubMed]
- Magalhaes MA, Minha S, Lhermusier T, et al. Does direct stenting with drug-eluting stents improve outcome? A meta-analysis of 10,900 patients. Catheter Cardiovasc Interv 2017;90:213-22. [Crossref] [PubMed]
- Ozdemir R, Sezgin AT, Barutcu I, et al. Comparison of direct stenting versus conventional stent implantation on blood flow in patients with ST-segment elevation myocardial infarction. Angiology 2006;57:453-8. [Crossref] [PubMed]
- Loubeyre C, Morice MC, Lefèvre T, et al. A randomized comparison of direct stenting with conventional stent implantation in selected patients with acute myocardial infarction. J Am Coll Cardiol 2002;39:15-21. [Crossref] [PubMed]
- Gasior M, Gierlotka M, Lekston A, et al. Comparison of outcomes of direct stenting versus stenting after balloon predilation in patients with acute myocardial infarction (DIRAMI). Am J Cardiol 2007;100:798-805. [Crossref] [PubMed]
- Dudek D, Mielecki W, Burzotta F, et al. Thrombus aspiration followed by direct stenting: a novel strategy of primary percutaneous coronary intervention in ST-segment elevation myocardial infarction. Results of the Polish-Italian-Hungarian RAndomized ThrombEctomy Trial (PIHRATE Trial). Am Heart J 2010;160:966-72. [Crossref] [PubMed]
- Mahmoud KD, Jolly SS, James S, et al. Clinical impact of direct stenting and interaction with thrombus aspiration in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention: Thrombectomy Trialists Collaboration. Eur Heart J 2018;39:2472-9. [Crossref] [PubMed]


