As the screening tests for coagulopathy diseases and monitoring approach for anticoagulants treatment, PT and APTT reflect the integrity of the extrinsic and endogenous pathways of the procoagulant cascade, respectively (
1). In this case, the abnormal prolonged PT and APTT seems not attributed to COPD, since previous reports has shown that the coagulation changes were small amount in COPD patients (
2). Further investigation revealed that the patient presented no bleeding symptoms, had no family history of bleeding disorder, as well as anticoagulants treatment histories. Therefore, we speculated the abnormalities of PT and APTT were attributed to analytical errors or pre-analytical errors. The former was excluded soon after we seriously analyzed the same specimen again, since both PT and APTT were similar with previous test results. To exclude the pre-analytical errors, such as test tubes’ quality, blood collection, specimen transportation and storage condition (
3), a new sample test was asked. According to the quality control requirements of blood coagulation experiment (
4), a new sample with serious collection, transportation and storage was analyzed, however, consistent with previous results; abnormal PT and APTT were observed. In summary, the tests results were insignificant regardless of the same specimen or new ones. Further, we hypothesis that the preexisting hematologic conditions, including hyperbilirubinemia, hyperlipidemia and hemolysis (
5), may explained the prolonged PT and APTT. However, this possibility was easily excluded, as for no visible hemolysis, hyperbilirubinemia, hyperlipidemia to naked eye was seen, and the laboratory test found neither hyperbilirubinemia nor hyperlipidemia.
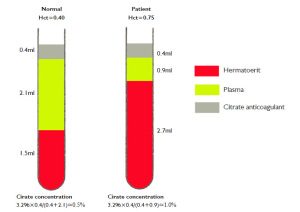
The main cause of the false prolongation was of the significantly increased hematocrit (Hct). In normal circumstances, a 3.2% citrate solution was used in the vacuum blood tubes prevent the activation. The mechanism under the anticoagulation effect of citrate was acted by their irreversible binding ability to plasma calcium, an essential element of procoagulant cascade (
6). For patients with typical hematocrits (35%-50%), the volume of anticoagulant to whole blood will be in the proper 1:9, and to plasma will be approximately 1:5 ratios (suppose the hematocrit was 40%) (
Figure 1, left). Thus, the final citrate concentration under this condition is approximately 0.5%. During PT and APTT testing, an additional calcium, often contain in the analytical reagent, is added to plasma to neutralize the anticoagulation effects of citrate, thus allowed plasma to clot in the present of extrinsic (phospholipids) or intrinsic (thromboplastin) pathway activator. In this case, due to impaired lung function caused by COPD, the patient's hematocrit was 75.3%, which markedly exceeded its upper reference limits. The volume ratio of anticoagulant to plasma was approximately 1:2, failed to fall into the proper 1:5 ratio (Figure 1, right). Under such condition, the citrate sodium would be 1.0%. Obviously, the excessive citrate sodium in plasma will weaken the procoagulant activity of PT and APTT reagent by reducing the availability of assay-added calcium and consequently result in an artifactual prolongation of PT and APTT.
According to CLSI guidelines, for patients with hematocrits of more than 55%, the ratio of anticoagulant and the plasma should be adjusted to yield more reliable results (
7). To correct the ratio, 0.20 ml of citrate was discarded from collection tube (the total volume of citrate in collection tube was 0.4 ml) before blood collection, and the results showed that PT and APTT values were 11.3 s and 35.7 s, respectively.