Changes of HMGB1 and sRAGE during the recovery of COPD exacerbation
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide, which is characterized by irreversible airflow limitation, and usually progressive and associated with abnormal inflammatory responses of the lung to noxious particles or gases (1). Despite the involvement of airway inflammation in the development of COPD, current anti-inflammatory therapies poorly prevent the deterioration of COPD, which indicate that more efforts are need to explore novel molecular mechanisms and targets involved in the disease pathogenesis and progression (2).
High mobility group protein B1 (HMGB1) is an abundant chromatin protein that acts as a cytokine when released into the extracellular milieu by necrotic and inflammatory cells. It is regarded as a marker of tissue injury and a mediator of inflammation. It has been reported that extracellular HMGB1 contributes to the pathogenesis of many inflammatory diseases, such as sepsis, acute lung injury, adult respiratory distress syndrome, cystic fibrosis and systemic lupus erythematosus (3). HMGB1 has been shown to transduce cellular signals by interacting with at least three receptors: the receptor for advanced glycation end-products (RAGE), toll-like receptors (TLR) 2/4. RAGE is the first identified receptor of HMGB1, binding of HMGB1 to cell surface RAGE results in generation of reactive oxygen species (ROS) and activation of the transcription factor NF-κB, which further promotes cytokines production, all these bioactivators stimulates immune and inflammatory responses (4).
RAGE belongs to the immunoglobulin superfamily of cell surface molecules. RAGE is expressed as both a transmembrane molecule and a soluble molecule. The soluble form seems to be produced by either alternative mRNA splicing or proteolytic cleavage of transmermbrane RAGE (mRAGE). Soluble RAGE (sRAGE) is capable of binding the same RAGE ligands, including HMGB1, it has been proposed that sRAGE acts as a decoy receptor, preventing the interaction of mRAGE with its ligands (5). In the majority of healthy mature tissues, RAGE is expressed at a low basal level, its expression is up-regulated at sites of various pathologies, such as diabetes, atherosclerosis and Alzheimer’s disease (5). Pulmonary tissues express relatively high basal levels of RAGE, especially in alveolar epithelial cells (6), suggesting that HMGB1/RAGE pathway may have a number of functions in the lung. Since sRAGE presumably inhibits the activity of HMGB1, it might have potential value in the clinic to treat some lung inflammatory diseases with high level of HMGB1.
Recent studies have reported an enhanced level of HMGB1 in BAL fluid (7), sputum and circulation in patients with COPD (8). Study has shown that the level of plasma sRAGE decreases in patients with COPD in comparison with healthy controls, and its level is even lower in acute exacerbation of COPD (AECOPD) (9), however, the data are less robust. There are fewer studies simultaneously investigating changes of HMGB1, sRAGE and the ratio of HMGB1/sRAGE in acute exacerbation and convalescence phase of COPD, and determining the correlation of the levels of HMGB1, sRAGE and ratio of HMGB1/sRAGE with clinical characteristics of COPD. This study aims to clarify these issues.
Methods
Subjects
The present prospective cohort study was approved by the Research Committee of Human Investigation of Medical College of Xi’an Jiaotong University. All participants were given informed consent. Patients’ main hospitalization diagnosis, medical history, and smoking history were collected by physicians. A total of 102 patients with AECOPD were admitted to the Department of Respiratory Medicine of the Second Affiliated Hospital of Medical College, Xi’an Jiaotong University (Xi’an, Shanxi, China) between October 2012 and March 2013. Fifty-eight COPD patients with coronary artery disease, diabetes, autoimmune disease and any other conditions which could affect HMGB1and sRAGE levels were excluded. Forty-four COPD patients were recruited into this study.
Clinical variables
Detailed clinical and demographic data were obtained at the time of hospital admission. Smoking history, hospitalization time, course of disease and exacerbation frequency in previous 3 years were also collected. Laboratory measurements including total leukocyte counts, neutrophils %, serum high-sensitivity C-reactive protein (hsCRP), plasma fibrinogen; arterial blood gas analyses and chest CT were performed.
Criteria for acute exacerbation and convalescence of COPD
AECOPD is defined as the worsening of respiratory symptoms, and is diagnosed based on the presence of an increase in any two major symptoms (dyspnoea, sputum purulence and sputum quantity), or an increase in one major and one minor symptom (wheeze, sore throat, cough and nasal congestion/discharge) for at least two consecutive days according to previously accepted criteria (10). Convalescence of COPD is diagnosed on clinical grounds with the following criteria: symptoms of patient with COPD return to the pre-exacerbation level variability, physician carefully assesses the patient and confirms that the individual is medically stable enough to leave the hospital, but patients are not unstable and prone to acute exacerbation again (11).
Therapy during hospitalization
The routine treatment for AECOPD during hospitalization was as follows: all patients diagnosed with AECOPD were supplemented with low flow oxygen, intravenous infusions of methylprednisolone 0.7-1.4 mg/kg per day for the first 3 to 5 days, and switching to nebulized budesonide (2 mg, 8 hourly) for the following 6 to 9 days. Antibiotics administration was adjusted based on the results of blood tests for inflammatory markers, sputum test, and signs of pneumonia. Intravenous infusions of aminophylline and expectorants were prescribed until patients with AECOPD condition were stable. Inhaled long-acting β2 agonists were prescribed for maintenance therapy upon discharge.
Pulmonary function tests
Spirometry was performed on each subject. Reversibility assessment was conducted in COPD patients by making them inhale a short-acting β2 agonist equivalent to 200 μg salbutamol. For hospitalized patients with acute exacerbations, spirometry was performed 12 to 17 days after the onset of exacerbation when the patients were stable enough to perform the spirometer maneuver.
Blood sample preparation
Blood was collected at the following two time points: within 24 hours of hospitalization; before discharge from hospital after treatment for 12-17 days in hospital. Blood was drawn and stored by the same researcher in the same department. Blood samples were collected aseptically in ethylenediamine tetraceticacid (EDTA)-anticoagulated tubes and stored at 4 °C. Samples were centrifuged at 1,000 g at 4 °C for 15 min, and plasma was separated and stored at –80 °C in aliquots of 1 mL until the measurements were taken.
Measurements of HMGB1, sRAGE, hsCRP and fibrinogen
Plasma HMGB1 and sRAGE levels were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits (KYM, Beijing, China). The detection limit of the kits is 0.1 ng/mL for HMGB1 and sRAGE. Each sample was run in duplicate and compared with a standard curve. The mean concentration was determined for each sample. Fibrinogen and hsCRP were measured immediately in clinic laboratory once blood plasma was acquired.
Statistical analyses
The Statistical Package for Social Sciences (SPSS), version 13.0, was used to analyze the data. Normality of distribution was examined with Kolmogorove-Smirnov test. Normally distributed data were expressed as mean ± standard deviation (SD). Non-normally distributed data were expressed as median (range). Normally distributed data were compared using paired t-tests between acute exacerbation and convalescence phase of COPD; non-normally distributed data were analyzed using wilcoxon signed Ranks test. Frequency data were compared with χ2 test. Correlation of changes of sRAGE and HMGB1 was examined by a Pearson’s correlation. The differences of HMGB1 and sRAGE among groups (never smoker, current smokers and ex-smokers) of COPD were analyzed using Mann-Whitney U test. Multiple linear regression analysis were used to assess the relationship between HMGB1/sRAGE and COPD disease status, gender, age, course of disease, smoking history, FEV1% pred.
Results
Patient characteristics
Table 1 summarizes the demographic characteristics of all subjects. A total of 44 patients were evaluated (32 males, 12 females; age 68.2±8.5 years). A total of 30 patients were current or former smokers, they are mostly male, with a consumption of 39.5±17.6 pack-years. The hospitalization time of AECOPD was 14 (range, 12-17) days, and frequency of acute exacerbation in the 3 previous years was 0.53±0.21 times/year.
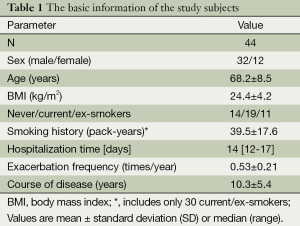
Full table
Laboratory parameters
Total leucocytes counts, neutrophils %, arterial blood gas analysis, hsCRP, fibrinogen were determined and recorded at the time of admission and discharge. Pulmonary function was not performed at the time of admission due to worsening of physical status, and was examined before patients discharge. The laboratory parameters of subjects are shown in Table 2.
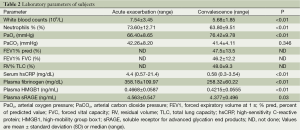
Full table
Plasma HMGB1, sRAGE, fibrinogen and serum hsCRP levels in exacerbation and convalescence phase of COPD
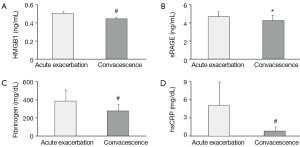
The changes of plasma HMGB1, sRAGE, fibrinogen and serum hsCRP level are shown in Table 2 and Figure 1A-D. There was a significant decline in plasma HMGB1 (P<0.01), sRAGE (P<0.05), fibrinogen (P<0.01) and serum hsCRP (P<0.01) level from acute exacerbation to convalescence phase in COPD.
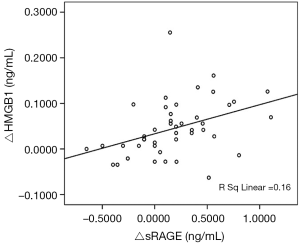
Correlation of the changes of sRAGE and HMGB1 in exacerbation and convalescence phase
The correlation between the change of HMGB1 level and the change of sRAGE level in acute exacerbation and convalescence is shown in Figure 2. There was a significant positive correlation between the change of sRAGE and change of HMGB1 (r=0.4, P=0.007).
HMGB1/sRAGE ratio correlates with COPD disease status
In the multivariate linear regression analysis with HMGB1/sRAGE as a dependent variable, COPD disease status was found to be an only major influence factor affecting HMGB1/sRAGE ratio (unadjusted r2=0.102, adjusted r2=0.036), whereas gender, age, course of disease, smoking history and FEV1% pred did not appear to be significant factors influencing HMGB1/sRAGE ratio (Table 3).
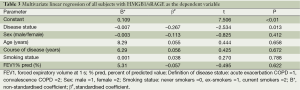
Full table
HMGB1 and sRAGE levels with smoking
Subjects with COPD were classified into three groups of never smoker, current smoker and ex-smoker according to smoking history. Table 4 shows that the level of HMGB1 was the highest in current smoker group, and decreased significantly in ex-smoker group in either acute exacerbation or convalescence phase. However, HMGB1 level was higher in never smoker group than ex-smoker group. The change of sRAGE level had a similar pattern.
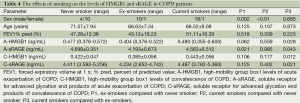
Full table
Discussion
The episode of COPD exacerbation is characterized by an increase of various inflammatory markers in lung, including neutrophils, macrophages and various cytokines (12). The activation of NF-κB plays a critical role in inflammatory responses of COPD exacerbation (13). Studies have shown that extracellular HMGB1 is a critical mediator in late stage of inflammatory responses, HMGB1 signals through RAGE leading to activation of NF-κB and subsequent up-regulation of various leukocyte adhesion molecules, membrane receptor and pro-inflammatory cytokines, such as IL-1β, IL-6, TNF-α and RAGE (14,15). These pro-inflammatory cytokines in turn promote hepatic synthesis of hsCRP and fibrinogen (12). High level of RAGE is expressed in alveolar epithelial cells and alveolar macrophages and is thought to be important in the pathogenesis of COPD (4). Studies have shown that plasma HMGB1 level in COPD patients is significantly greater than that in healthy controls. We showed here that HMGB1 was also associated with the occurrence of AECOPD, its level raised in the AECOPD, and declined in convalescence phase of COPD. It seems that HMGB1 may be not only a potential inflammatory marker in COPD but also a therapeutic target for COPD.
sRAGE corresponds to the extracellular domain of membrane-bound RAGE lacking its cytosolic and transmembrane domains, and exists in extracellular fluids. sRAGE may act as a decoy receptor binding RAGE ligands and preventing them binding to membrane RAGE (5). Numerous studies have reported that a correlation between sRAGE level and inflammatory diseases, for example, sRAGE level was elevated in BAL fluid and plasma in patients with acute lung injury, it was positively correlated with the severity of acute lung injury (16). In our study, we also observed that the level of plasma sRAGE was higher in AECOPD than that of convalescence. These results seem inconsistent with the notion that sRAGE functions to decoy HMGB1. Study by Smith et al. suggested that sRAGE was lower in stable COPD than in healthy control, and AECOPD was associated with even lower sRAGE level, sRAGE increased with COPD convalescence (9). This discrepancy may be due to several reasons. In Smith’s study, the comorbidities of patients with AECOPD and oxygen therapy in these patients were not counted and analyzed. AECOPD is associated with worsening hypoxia and activation of NF-κB, which increases expression of membrane RAGE (17). Soluble RAGE is generated by proteolytic cleavage of membrane-bound RAGE (18), this process is mediated by disintegrin, metalloprotease (ADAM10) (19) and matrix metalloproteinase-9 (MMP-9) (20). It has also been shown that MMP-9 activity and ADAM10 expression are increased in AECOPD compared to convalescence of COPD (21-23), which stimulate membrane RAGE shedding and promote the release of sRAGE (20). In addition, HMGB1 has also been found to induce RAGE shedding (24).
The results of the present study suggested that gender, age, course of disease, smoking history and FEV1% pred did not significantly affect HMGB1/sRAGE ratio. COPD disease status was found to be an only major factor affecting HMGB1/sRAGE ratio. The ratio of HMGB1/sRAGE was elevated in exacerbation of COPD and declined in convalescence of COPD, suggesting that although both levels of HMGB1 and sRAGE were increased in AECOPD, the elevation of HMGB1 was predominant. Our study further indicated that the levels of HMGB1 and sRAGE in circulation were the highest in currently smoking COPD patients, and the lowest levels were in ex-smoker COPD patients. We inferred that smoking can induce the elevation of HMGB1 levels, which were declined by quitting smoking. Cigarette smoke was an extremely concentrated sources of ROS and reactive nitrogen species (25), which activates inflammasomes leading to releasing HMGB1 (26). As mentioned above, HMGB1 stimulate RAGE shedding and promote the release of sRAGE, the trend of sRAGE level had a similar with HMGB1. However, the reasons for levels of HMGB1 and sRAGE higher in never smoking COPD than ex-smoking COPD were still unclear.
Limitations of this study include small sample size, lack of measurement of lung function prior to treatment of AECOPD, and lack of complete long-term follow-up. Despite such limitations, this study provides valuable preliminary information about changes of HMGB1 and sRAGE in COPD exacerbation episodes.
Conclusions
The present study showed that the levels of plasma HMGB1, sRAGE, fibrinogen and hsCRP were elevated in AECOPD and tended to decline in convalescence of COPD; the ratio of HMGB1/sRAGE was correlated with status COPD. Meanwhile, the reduction of plasma HMGB1 was correlated with the reduction of plasma sRAGE from acute exacerbation phase to convalescence phase. The concentrations of plasma HMGB1 and sRAGE in patients with COPD were influenced by smoking.
Acknowledgements
Funding: This work was supported by the Key Clinical Project for Affiliated Hospital of Ministry of Public Health of China (No. 111).
Disclosure: The authors declare no conflict of interest.
References
- Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet 2012;379:1341-51. [PubMed]
- Kim V, Rogers TJ, Criner GJ. New concepts in the pathobiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008;5:478-85. [PubMed]
- Nogueira-Machado JA, de Oliveira Volpe CM. HMGB-1 as a target for inflammation controlling. Recent Pat Endocr Metab Immune Drug Discov 2012;6:201-9. [PubMed]
- Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 2005;5:331-42. [PubMed]
- Maillard-Lefebvre H, Boulanger E, Daroux M, et al. Soluble receptor for advanced glycation end products: a new biomarker in diagnosis and prognosis of chronic inflammatory diseases. Rheumatology (Oxford) 2009;48:1190-6. [PubMed]
- Demling N, Ehrhardt C, Kasper M, et al. Promotion of cell adherence and spreading: a novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell Tissue Res 2006;323:475-88. [PubMed]
- Ferhani N, Letuve S, Kozhich A, et al. Expression of high-mobility group box 1 and of receptor for advanced glycation end products in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010;181:917-27. [PubMed]
- Hou C, Zhao H, Liu L, et al. High mobility group protein B1 (HMGB1) in Asthma: comparison of patients with chronic obstructive pulmonary disease and healthy controls. Mol Med 2011;17:807-15. [PubMed]
- Smith DJ, Yerkovich ST, Towers MA, et al. Reduced soluble receptor for advanced glycation end-products in COPD. Eur Respir J 2011;37:516-22. [PubMed]
- Vestbo J, Hurd SS, Rodriguez-Roisin R, et al. An overview of Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (GOLD) (revised 2011). Zhonghua Yi Xue Za Zhi 2012;92:937-8. [PubMed]
- Koutsokera A, Kiropoulos TS, Nikoulis DJ, et al. Clinical, functional and biochemical changes during recovery from COPD exacerbations. Respir Med 2009;103:919-26. [PubMed]
- Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA 2013;309:2353-61. [PubMed]
- Di Stefano A, Caramori G, Oates T, et al. Increased expression of nuclear factor-kappaB in bronchial biopsies from smokers and patients with COPD. Eur Respir J 2002;20:556-63. [PubMed]
- Huttunen HJ, Rauvala H. Amphoterin as an extracellular regulator of cell motility: from discovery to disease. J Intern Med 2004;255:351-66. [PubMed]
- Li J, Schmidt AM. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J Biol Chem 1997;272:16498-506. [PubMed]
- Jabaudon M, Futier E, Roszyk L, et al. Soluble form of the receptor for advanced glycation end products is a marker of acute lung injury but not of severe sepsis in critically ill patients. Crit Care Med 2011;39:480-8. [PubMed]
- Tafani M, Schito L, Pellegrini L, et al. Hypoxia-increased RAGE and P2X7R expression regulates tumor cell invasion through phosphorylation of Erk1/2 and Akt and nuclear translocation of NF-{kappa}B. Carcinogenesis 2011;32:1167-75. [PubMed]
- Hudson BI, Carter AM, Harja E, et al. Identification, classification, and expression of RAGE gene splice variants. FASEB J 2008;22:1572-80. [PubMed]
- Raucci A, Cugusi S, Antonelli A, et al. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J 2008;22:3716-27. [PubMed]
- Zhang L, Bukulin M, Kojro E, et al. Receptor for advanced glycation end products is subjected to protein ectodomain shedding by metalloproteinases. J Biol Chem 2008;283:35507-16. [PubMed]
- Gao P, Zhang J, He X, et al. Sputum inflammatory cell-based classification of patients with acute exacerbation of chronic obstructive pulmonary disease. PLoS One 2013;8:e57678. [PubMed]
- Zeng M, Wen Y, Liu LY, et al. Role of TNF-α, sTNF-R55 and sTNF-R75 in inflammation of acute exacerbations of chronic obstructive pulmonary disease. Respiration 2009;78:399-403. [PubMed]
- Zhu LB, Zhao ST, Xu TZ, et al. Tumor necrosis factor-α-induced a disintegrin and metalloprotease 10 increases apoptosis resistance in prostate cancer cells. Oncol Lett 2014;7:897-901. [PubMed]
- Sugaya K, Fukagawa T, Matsumoto K, et al. Three genes in the human MHC class III region near the junction with the class II: gene for receptor of advanced glycosylation end products, PBX2 homeobox gene and a notch homolog, human counterpart of mouse mammary tumor gene int-3. Genomics 1994;23:408-19. [PubMed]
- Churg A, Cosio M, Wright JL. Mechanisms of cigarette smoke-induced COPD: insights from animal models. Am J Physiol Lung Cell Mol Physiol 2008;294:L612-31. [PubMed]
- Lamkanfi M, Sarkar A, Vande Walle L, et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J Immunol 2010;185:4385-92. [PubMed]


