
Spontaneous coronary artery dissection: the great pretender
We read with great interest the recent publication by Alfonso et al. (1) published in the July 2018 issue of Journal of Thoracic Disease. Spontaneous coronary artery dissection (SCAD) usually affects young and otherwise healthy women, mainly in the postpartum period. Incidence rates from 0.2% to 1.1% are reported (1,2). Prevalence is estimated as high as 8.7% (3) among women <50 years old with acute coronary syndromes (ACS). Clinical presentation varies from isolated chest pain to ST-segment elevation myocardial infarction, ventricular fibrillation, and sudden death. Treatment can be pharmacological and percutaneous or surgical myocardial revascularization, depending on location and extent of the lesions. In this report, we sought to highlight the complex decision process performed by the IRCCS-ISMETT multidisciplinary team and involving clinical and interventional cardiologists, radiologists and cardiac surgeons in the management of a patient with SCAD. Moreover, both coronary computed tomography angiography (CCTA) and cardiac magnetic resonance imaging (CMRI) are often required in order to establish the definition of coronary anatomy, dissection layer and ongoing myocardial ischemia. Critical clinical judgment is also crucial for the best surgical timing. Surgical treatment, whenever required, can be challenging in term of coronary reconstruction and maintenance of coronary perfusion. Availability of extracorporeal membrane oxygenation, in case of acute cardiogenic shock, is also advocated.
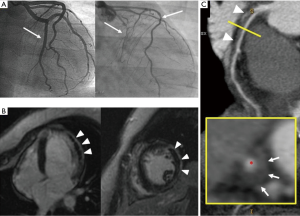
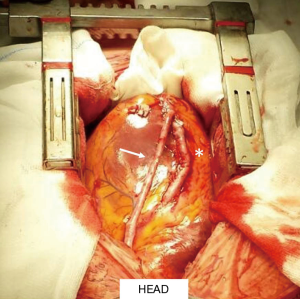
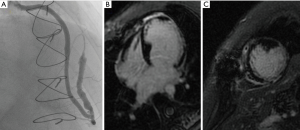
We present a case of ACS occurring 2 weeks after delivery in a 34-year-old woman. At presentation, she had elevated troponin I levels without electrocardiogram (ECG) and echocardiogram abnormalities, normal coronary angiography (CA) (Figure 1A, left side). Intravenous nitrates and diltiazem were started. Two days later, intense chest pain with infero-postero-lateral ST elevation recurred. Left ventricular ejection fraction (LVEF) was 25%, with apical and postero-lateral akinesia. CA showed a type 2B SCAD, involving mid-distal circumflex (CX), with intramural hematoma appearance. Proximal-mid tract of the left anterior descending (LAD) coronary artery was mildly stenotic (Figure 1A, right side). CMRI showed moderate LVEF reduction, diffuse hyperenhanced signal consistent with edema on the vascular territory of the CX, with an area of transmural delayed enhancement (DE). An extensive area of microvascular obstruction was also seen (Figure 1B). CCTA revealed extensive dissection of CX and LAD (Figure 1C), with intramural hematoma and severe luminal narrowing (*). Considering the large amount of myocardium at risk, persistence of intractable angina, and cardiogenic shock, urgent surgical revascularization was decided. After pericardiotomy, an extended intramural hematoma of LAD and CX and severely impaired and distended left ventricle was identified. A long arteriotomy was performed on the LAD, following its course on the epicardial surface up to the cardiac apex. A saphenous vein patch was directly anastomosed on LAD using an 8/0 mono-filamentous running suture connecting the intima and adventitia layers in order to redirect the flow into the true lumen. After LAD reconstruction, a coronary artery bypass graft (CABG) with a segment of reversed saphenous vein graft was performed on the patch (Figure 2). The CX was not treated because of absence of viable myocardium in its territory of perfusion. Post-operative course was uneventful, and the woman was discharged 14 days later in dual antiplatelet therapy (aspirin and clopidogrel), NYHA class II. Post-operative CA documented thrombolysis in myocardial infarction (TIMI 3 flow) of venous graft on distal reconstructed LAD (Figure 3A); LVEF was 35%. At follow-up, CMRI documented ischemic area with subendocardial DE on mid-apical interventricular sept (Figure 3B,C).
SCAD is an infrequent, under-recognized, potentially deadly condition. The etiology is not completely understood and seems multifactorial; fibromuscular dysplasia, pregnancy, connective tissue disorders, and systemic inflammatory diseases are predisposing factors (4). Intense exercise or emotional stress, labor and delivery, and hormonal therapy can act as precipitators (5). The characteristic angiographic double lumen flap is the cornerstone of diagnosis. Subtler forms might be difficult to distinguish from vasospasm or atherosclerosis (4). Integration with intravascular ultrasound (IVUS) and optical coherence tomography (OCT) can, respectively, delineate true from false lumens and visualize intimal tearing. Treatment is not well established: hemodynamic profile of the patient, site of dissection, and number of vessels involved can guide the approach. Conservative management consists of heparin, b-blockers, calcium channel blockers, diuretics, and antiplatelet therapy. This approach is applied in mid or distal single vessel dissection with a lumen diameter limitation of <50%, with TIMI flow 2–3 (6). On the other hand, there is evidence that early revascularization, either percutaneous or surgical, can lead to a better outcome (7). PCI is used mainly in the single vessel scenario, with ongoing symptoms, and persistent restriction of blood flow. Nevertheless, it carries a high risk of propagation of dissection (8). CABG could be considered in the case of left main involvement, extensive dissections of proximal and/or multiple coronary arteries, and in the case that percutaneous coronary intervention (PCI) is not feasible or has failed (9). In our case, extensive LAD involvement and cardiogenic shock rendered a surgical approach mandatory, as recently described (5). In similar scenarios, 1-year mortality is 1–4% (6). Long-term recurrence is 27% (7), with a 10-year rate of major cardiac events of 50% (9).
In conclusion, as far as we know, this is the first report of SCAD treated with this surgical approach. High clinical suspicion helps to timely diagnose SCAD in susceptible patient populations. Given the lack of any standard guidelines, case-based selection of available treatment options can improve outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Alfonso F, García-Guimaraes M, Bastante T, et al. Spontaneous coronary artery dissection: from expert consensus statements to evidence-based medicine. J Thorac Dis 2018;10:4602-8. [Crossref] [PubMed]
- Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012;126:579-88. [Crossref] [PubMed]
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol 2013;29:1027-33. [Crossref] [PubMed]
- Alfonso F, Bastante T, Rivero F, et al. Spontaneous coronary artery dissection. Circ J 2014;78:2099-110. [Crossref] [PubMed]
- Saw J, Mancini GBJ, Humphries KH. Contemporary Review on Spontaneous Coronary Artery Dissection. J Am Coll Cardiol 2016;68:297-312. [Crossref] [PubMed]
- Vanzetto G, Berger-Coz E, Barone-Rochette G, et al. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg 2009;35:250-4. [Crossref] [PubMed]
- Tweet MS, Eleid MF, Best PJ, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv 2014;7:777-86. [Crossref] [PubMed]
- Arnold JR, West NE, van Gaal WJ, et al. The role of intravascular ultrasound in the management of spontaneous coronary artery dissection. Cardiovasc Ultrasound 2008;6:24. [Crossref] [PubMed]
- Lettieri C, Zavalloni D, Rossini R, et al. Management and Long-Term Prognosis of Spontaneous Coronary Artery Dissection. Am J Cardiol 2015;116:66-73. [Crossref] [PubMed]


