Minimally invasive and robotic Ivor Lewis esophagectomy
Introduction
Esophageal cancer is the eighth most common malignancy and the sixth most common cause of cancer-related death in the world. An estimated 482,300 new cases and 406,800 cancer deaths occurred in 2008 worldwide (1), showing a high mortality-to-incidence rate ratio of 0.84. Incidence rates vary internationally, and China has the fourth highest rate of esophageal cancer according to the GLOBOCAN 2008 Database. In the United States, approximately 17,990 patients are diagnosed with esophageal cancer in 2013 with a mortality of 15,210 (2). The overall 5-year survival rate for esophageal cancer remains poor, despite the modest improvement from 5% between 1975 and 1977 to 19% between 2002 and 2008 (2). Several surgical techniques are available, and the choice of technique depends on tumor location, extent of lymphadenectomy, the patient’s overall condition and surgeon’s preference. The two most frequent open techniques are transhiatal esophagectomy (THE) and transthoracic esophagectomy (TTE). THE involves laparotomy with blunt dissection of the esophagus (without thoracotomy) and cervical esophagogastric anastomosis (3). Ivor Lewis esophagectomy (ILE) is the classic TTE, which consists of laparotomy and right thoracotomy with intrathoracic anastomosis (4). The 3-incision McKeown approach is a modified TTE, which utilizes the right thoracic and abdominal portions of ILE with an added left cervical anastomosis. Compared to THE, TTE allows the removal of the intrathoracic esophageal tumor with a wider radial margin, and the oncologic resection of extensive mediastinal lymph nodes (5), but is associated with significant in-hospital morbidity (but not mortality), predominantly respiratory complications (6,7). THE carries a lower complication rate, but only a limited lymphadenectomy can be performed with no dissection of the carinal and paratracheal lymph nodes (6,7). Although no significant difference in 5-year survival was seen between the THE and TTE groups, there was a trend towards survival benefit: overall survival was 29% in the THE group, as compared with 39% in the TTE group (6).
To reduce the surgical morbidity and mortality, multiple minimally invasive approaches have been explored in esophagectomy. Several studies have shown a substantial decrease in blood loss, complication rate and hospital stay when minimally invasive esophagectomy (MIE) was applied (8,9). However, MIE has several intrinsic limitations, including 2-dimensional view, reduced eye-hand coordination and a decrease in degrees of freedom of movement (10). These may create difficulties in mediastinal dissection and anastomosis during thoracoscopic esophagectomy. Robotic systems have been designed to overcome some disadvantages of standard minimally invasive surgery. The da Vinci® robotic system (Intuitive Surgical, Inc. California, USA) provides a magnified 3-dimensional vision system and special wristed instruments that offer more degrees of freedom (10). It translates the surgeon’s hand movement into precise real-time movements of surgical instruments, filters the tremor and restores the natural eye-hand coordination. These technical improvements facilitate precise dissection in a confined operating filed, and may benefit mediastinal dissection of esophagus and surrounding lymph nodes.
This article reviews development and techniques of minimally invasive ILE (MI-ILE), and introduces robotics in the management of esophageal cancer.
Minimally invasive Ivor Lewis esophagectomy (MI-ILE)
The conventional ILE consists of a laparotomy and a right thoracotomy for esophageal resection (and lymphadenectomy) followed by an intrathoracic anastomosis of the gastric conduit with the proximal esophagus at the level of the proximal mediastinum (4). The following components of ILE may differ from surgeon to surgeon: technique of pyloric drainage (pyloromyotomy versus pyloroplasty versus Botox injection versus none); inclusion of jejunostomy; width of the gastric tube; technique of anastomosis (mechanical versus hand sewn). The advantages of ILE include excellent visualization of all parts of the operation, ability to perform 2-field lymphadenectomy, and potential prevention of cervical dissection of the esophagus and consequent complications, such as stenosis, leakage and recurrent laryngeal nerve injury. The disadvantages are the need for single lung ventilation, morbidity associated with a thoracotomy, higher risk for respiratory complications, and the potential danger caused by a postoperative anastomotic leak (11).
To reduce surgical trauma and overcome some of the disadvantages, various minimally invasive approaches have been explored in ILE, including any combination of laparoscopy instead of laparotomy, thoracoscopy instead of thoracotomy and intrathoracic anastomosis. Watson et al. first described a totally endoscopic ILE in two patients, which incorporated a hand-assisted laparoscopy for gastric mobilization and a right thoracoscopy for esophageal dissection and anastomosis (12). Nguyen et al. then reported a series of three patients receiving a completely MI-ILE of combined laparoscopic and thoracoscopic resection of the distal esophagus with an intrathoracic anastomosis reconstruction (13,14). All patients had an uneventful postoperative course. In 2006, Bizekis and colleagues described their experience in 50 patients who underwent MI-ILE from 2002 to 2005 (15). Thirty five patients (70%) underwent a hybrid ILE (laparoscopic gastric mobilization combined with a minithoracotomy); the remainder (30%) had a completely MI-ILE (laparoscopy and thoracoscopy). A circular stapled anastomosis was performed in all patients. The operative mortality rate was 6% (3/50). Three patients (6%) developed an anastomotic leak; all were successfully managed nonoperatively. Four patients (8%) developed postoperative pneumonia (15). There were no recurrent laryngeal nerve injuries. They concluded that a MI-ILE is technically feasible. MI-ILE approach could minimize the gastric mobilization, avoid recurrent laryngeal nerve injury, and allow a more extensive gastric resection in the case of cardia extension of gastroesophageal junction tumors (15). Similarly, Nguyen and coworkers later reported a series of 104 MIE procedures performed between 1998 and 2007, in which 51 cases were MI-ILE and 47 cases were combined laparoscopic and thoracoscopic McKeown esophagectomy (MI-McKeown, cervical anastomosis) (16). In the MI-ILE group, the mortality rate was 1.96% (1/51) and leak rate was 9.8%, which was comparable to the other group. Interestingly, the MI-ILE group had significant shorter operative time and less blood loss (16). They again showed MIE is feasible with acceptable morbidity and low mortality. They also preferred MI-ILE due to the important advantages of constructing a tension-free intrathoracic anastomosis and the ability to resect the tip of the gastric conduit (16). Other groups also reported successful completion of MI-ILE procedures with comparable outcomes (17-24). In a recent review of Luketich et al., they compared the results of 481 patients undergoing MIE-McKeown to 530 patients undergoing MI-ILE (25). Both approaches resulted in acceptable lymph node resection, postoperative outcomes and low mortality. They proposed MI-ILE as their preferred approach because it was associated with decreased recurrent laryngeal nerve injury and mortality rate of 0.9%.
Techniques of the MI-ILE
As pioneers in MIE, Luketich and the Pittsburgh group described the modified MI-ILE procedures in recent publications (25-27). For the laparoscopic portion of the procedure, the patient is initially positioned in a steep reverse Trendelenburg position, and a double lumen endotracheal tube is placed in preparation for the later thoracoscopic stage. Five abdominal ports are used. A 10-12 mm port is first placed via a Hasson technique in the epigastrium between the xiphoid and umbilicus to the right of midline. Subsequent ports are placed under direct laparoscopic visualization. A 5 mm camera port is placed just to the left of the midline at the same level as the 10 mm port. Two additional 5 mm ports are inserted at the right and left subcostal margins. The final 5 mm port is placed at the right flank for liver retractor. After an abdominal inspection to rule out advance disease, the gastrohepatic ligament is divided. The exposed right crus is dissected, followed by dissection of the left crus until the gastroesophageal junction is freed. The greater curvature of the stomach is mobilized by dividing the short gastric vessels using the ultrasonic coagulation shears. The gastrocolic omentum is then divided, with care taken to preserve the right gastroepiploic arcade. Posterior gastroesophageal attachments are divided after retraction of the stomach anteriorly. A complete celiac node dissection can be performed before division of the left gastric vessels with a vascular stapler. Next, Luketich et al. perform a pyloroplasty whereas some other groups do not. A gastric tube is created with a stapling device from the lesser curvature towards the fundus of stomach, preserving the right gastric vessels. There are some variations regarding the diameter of the gastric tube. Luketich et al. reported an increase of ischemia and high leak rate with a too narrow tube (3-4 cm in diameter), and hence they emphasized the importance of creating a gastric tube of 5-6 cm in diameter (8). Berrisford et al. also observed a high gastric tube ischemia and leak rate by using a 4 cm gastric tube (28). Currently, creating a 5 cm wide gastric tube is recommended in MIE by Wee and Morse (29). Next, a jejunostomy tube is placed before division of the phrenoesophageal membrane. The abdomen is inspected and the incisions are closed.
In the thoracoscopic phase, the patient is placed in a left lateral decubitus position. The position of the double-lumen tube is verified, and single-lung ventilation is used. In our hands, three thoracoscopic ports are used (Figure 1). A 10 mm camera port is placed in the eighth intercostal space, just posterior to the posterior axillary line. Access incisions are placed in the 5th and 10th/11th intercostal spaces. After division of the inferior pulmonary ligament, the mediastinal pleura is divided up to the level of the azygous vein to expose the thoracic esophagus, and the vein is divided with an endovascular stapler. The esophagus is circumferentially mobilized from the diaphragm to the level about 2 cm above the carina, and a Penrose drain is placed around it. Mediastinal lymph node dissection is performed. The distal esophagus and previously constructed gastric conduit are brought up into the chest. The proximal esophagus is then transected above the azygous vein. The eighth posterior interspace port is enlarged to 5 cm to remove specimen and complete construction of intrathoracic anastomosis. The redundant portion of the gastric conduit is then excised with endostapler and the thoracic cavity is drained. There are various intrathoracic anastomotic techniques in MI-ILE, including handsewn and stapled techniques. The stapled techniques varied with regard to transthoracic circular stapled, transoral circular stapled and side-to-side liner stapled. Anastomotic leak rates ranged from 0% to 10%, and anastomotic stenosis rates ranged from 0% to 27.5% (30).
MI-ILE outcomes
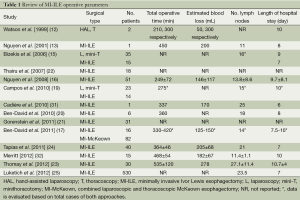
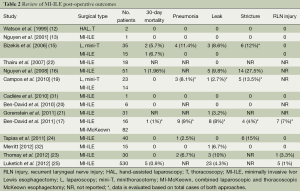
As with many novel procedures, the initial publications involving MI-ILE were mostly institutional series. Operative parameters, including operating time, estimated blood loss, number of lymph nodes harvested and length of hospital stay, were evaluated in MI-ILE (Table 1). Post-operative mortality and major complications of MI-ILE were also reviewed in Table 2. Theoretically, obviating the need of the thoracotomy, laparotomy, or both may reduce surgical pain, wound infections, cardiopulmonary complications, intensive care unit and hospital stays, and mortality rates. Although MI-ILE has been shown to be safe and feasible, a clear advantage with MI-ILE over conventional ILE has not been demonstrated. The ultimate answer to this important question is complicated by the lack of well-designed trials, the small number of institutional series, publications bias of satisfactory outcomes and the technical variations. Recently, there are several studies aiming to compare open transthoracic with MIE (33-36) (Tables 3,4). Patients in both groups underwent similar pre-operative and post-operative protocols. Operative data and post-operative data were collected. These studies demonstrate the feasibility and safety of MI-ILE, and show its potential of reducing blood loss, pulmonary complications and length of hospital stay. Prospective multi-center, randomized and controlled studies would be needed to draw definite conclusions.
Full table
Full table
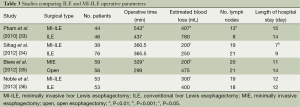
Full table
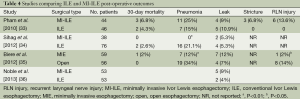
Full table
Another controversial issue with MI-ILE is whether its long-term survival rate is comparable with the open procedure, because the extent of lymphadenectomy may be compromised. Many series did not report on lymph node dissention, and the quality of lymph node dissection is difficult to evaluate. From the studies comparing open and MIE (Table 3), lymph node dissection is comparable between two groups. However, most of the major complications of MI-ILE were described within the perioperative period, and the long-term survival and disease progression data from large patient cohorts is absent (Table 4). Therefore, the potential of MI-ILE may not have been fully realized.
Robotic ILE
Some limitations of the minimally invasive approaches to esophagectomy include the 2-dimensional view, decreased freedom of movement, narrow field of the mediastinum and reduced eye-hand coordination. Robotic system provides the possibility to overcome some of these limitations by offering 3-dimensional camera with 10× magnification and wristed instruments (37). The robotic system can be used during the thoracic dissection of the esophagus, gastric mobilization and intrathoracic anastomosis. It can also be used in combination with laparoscopy, hand-assisted laparoscopy or thoracoscopy. Several groups have reported their early experience with robot-assisted ILE (38-40).
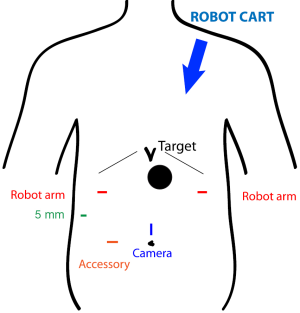
At our institution, we have begun to utilize the robotic system with MI-ILE. Figure 2 illustrates the port placement for the robotic abdominal procedure. The patient is placed in the supine position. A camera port is placed above the umbilicus, and a 12 mm accessory port is placed to the right of umbilicus. A liver retractor is placed through a 5 mm port in the low right subcostal space. Two additional ports for robot arms are placed in the right and left subcostal space at least a handbreadth from the camera port. The robotic cart comes over the patient’s left shoulder. The abdominal operation for gastric mobilization, gastric tube construction and jejunostomy tube placement is performed as described in MI-ILE procedure. In the robotic thoracoscopic stage, the patient is turned to the left lateral decubitus position and the right lung is deflated. Chest port placement is shown in Figure 3. The camera port is placed in the eighth intercostal space, posterior to the posterior axillary line. One robot instrument port is placed a handbreadth superior and a handbreadth anterior to the camera port. The other robot port is placed a handbreadth inferior and a handbreadth posterior to the camera port. A 5 mm port is placed between superior incisions, and a 12 mm port is placed between inferior incisions. The robotic cart comes over the patient’s right shoulder posteriorly. The thoracic operation for esophageal mobilization, lymphadenectomy and intrathoracic anastomosis is performed as in the above-mentioned MI-ILE procedure. However, we have preferred to use a stapled side-to-side anastomosis using an endoGIA stapler (45 mm purple load) and then to oversew the resulting defect with two layers of running suture (using the wristed robotic instruments).
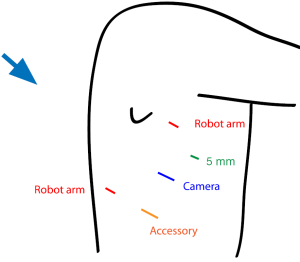
Robotic ILE outcomes
As a relatively new technology, data regarding the safety and the oncologic efficacy of robotic ILE are limited. de la Fuente et al. reported their initial experience with robotic ILE in 50 patients, which were comparable to open ILE and MI-ILE approaches (39): the mean operative time was 445±85 min. The estimated blood loss was 146±15 mL. The mean number of lymph nodes retrieved during surgery was 20±1.4. The mean length of hospitalization was 10.9±6.2 days. Mortality was 0 and main postoperative complications included pneumonia (10%) and anastomosis leak (2%). Study of Cerfolio et al. described similar results in 22 patients with robotic ILE with 40 mL blood loss, 18 lymph nodes harvested, 7 days of hospitalization, 0% mortality, and 4.5% anastomosis leak (40). These data suggest robotic ILE is safe, feasible and associated with perioperative outcomes similar to open ILE and MI-ILE. However, no evidence to date demonstrates improved outcomes of robotic over MI-ILE. The cost of equipment, specialized training, prolonged set up time and limited instrumentation are barriers to more widespread use. The fact that the surgeon is separated from the patient and the lack of tactile feedback raise potential safety concerns. For this procedure to be ultimately widely adopted, future studies are needed to prove identifiable benefit of robotic ILE relative to other approaches to offset inherent disadvantages and financial concerns.
Conclusions
MI-ILE has proven to have equivalent postoperative outcomes to open ILE, and thus represent a safe and feasible alternative for the surgical management of esophageal cancer. It also shows potential to reduce blood loss, postoperative pain and length of hospitalization. Improved long-term survival has not been documented in MI-ILE compared to conventional ILE. Prospective and randomized controlled trials comparing open ILE with MI-ILE are necessary if a definite conclusion is to be made about the superiority of one surgical technique over the other. Robotic approach may offer advantages to MI-ILE over conventional procedure. Further studies of MI-ILE and robotic ILE are warranted to determine the ideal esophagectomy procedure.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Turner GG. Carcinoma of the Esophagus: the Question of Its Treatment By Surgery. Lancet 1936;227:130-4.
- Lewis I. The surgical treatment of carcinoma of the oesophagus; with special reference to a new operation for growths of the middle third. Br J Surg 1946;34:18-31. [PubMed]
- Mathisen DJ, Grillo HC, Wilkins EW Jr, et al. Transthoracic esophagectomy: a safe approach to carcinoma of the esophagus. Ann Thorac Surg 1988;45:137-43. [PubMed]
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [PubMed]
- Hulscher JB, Tijssen JG, Obertop H, et al. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg 2001;72:306-13. [PubMed]
- Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg 2003;238:486-94; discussion 494-5. [PubMed]
- Gemmill EH, McCulloch P. Systematic review of minimally invasive resection for gastro-oesophageal cancer. Br J Surg 2007;94:1461-7. [PubMed]
- Camarillo DB, Krummel TM, Salisbury JK Jr. Robotic technology in surgery: past, present, and future. Am J Surg 2004;188:2S-15S. [PubMed]
- Reed CE. Technique of Open Ivor Lewis Esophagectomy. Operative Techniques in Thoracic and Cardiovascular Surgery 2009;14:160-75.
- Watson DI, Davies N, Jamieson GG. Totally endoscopic Ivor Lewis esophagectomy. Surg Endosc 1999;13:293-7. [PubMed]
- Nguyen NT, Follette DM, Lemoine PH, et al. Minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg 2001;72:593-6. [PubMed]
- Nguyen NT, Roberts P, Follette DM, et al. Thoracoscopic and laparoscopic esophagectomy for benign and malignant disease: lessons learned from 46 consecutive procedures. J Am Coll Surg 2003;197:902-13. [PubMed]
- Bizekis C, Kent MS, Luketich JD, et al. Initial experience with minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg 2006;82:402-6; discussion 406-7. [PubMed]
- Nguyen NT, Hinojosa MW, Smith BR, et al. Minimally invasive esophagectomy: lessons learned from 104 operations. Ann Surg 2008;248:1081-91. [PubMed]
- Ben-David K, Sarosi GA, Cendan JC, et al. Decreasing morbidity and mortality in 100 consecutive minimally invasive esophagectomies. Surg Endosc 2012;26:162-7. [PubMed]
- Ben-David K, Rossidis G, Zlotecki RA, et al. Minimally invasive esophagectomy is safe and effective following neoadjuvant chemoradiation therapy. Ann Surg Oncol 2011;18:3324-9. [PubMed]
- Campos GM, Jablons D, Brown LM, et al. A safe and reproducible anastomotic technique for minimally invasive Ivor Lewis oesophagectomy: the circular-stapled anastomosis with the trans-oral anvil. Eur J Cardiothorac Surg 2010;37:1421-6. [PubMed]
- Ben-David K, Sarosi GA, Cendan JC, et al. Technique of minimally invasive Ivor Lewis esophagogastrectomy with intrathoracic stapled side-to-side anastomosis. J Gastrointest Surg 2010;14:1613-8. [PubMed]
- Gorenstein LA, Bessler M, Sonett JR. Intrathoracic linear stapled esophagogastric anastomosis: an alternative to the end to end anastomosis. Ann Thorac Surg 2011;91:314-6. [PubMed]
- Thairu N, Biswas S, Abdulaal Y, et al. A new method for intrathoracic anastomosis in laparoscopic esophagectomy. Surg Endosc 2007;21:1887-90. [PubMed]
- Thomay AA, Snyder JA, Edmondson DM, et al. Initial results of minimally invasive Ivor Lewis esophagectomy after induction chemoradiation (50.4 gy) for esophageal cancer. Innovations (Phila) 2012;7:421-8. [PubMed]
- Tapias LF, Morse CR. A preliminary experience with minimally invasive Ivor Lewis esophagectomy. Dis Esophagus 2012;25:449-55. [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [PubMed]
- Levy RM, Wizorek J, Shende M, et al. Laparoscopic and thoracoscopic esophagectomy. Adv Surg 2010;44:101-16. [PubMed]
- Pennathur A, Awais O, Luketich JD. Technique of minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg 2010;89:S2159-62. [PubMed]
- Berrisford RG, Wajed SA, Sanders D, et al. Short-term outcomes following total minimally invasive oesophagectomy. Br J Surg 2008;95:602-10. [PubMed]
- Wee JO, Morse CR. Minimally invasive Ivor Lewis esophagectomy. J Thorac Cardiovasc Surg 2012;144:S60-2. [PubMed]
- Maas KW, Biere SS, Scheepers JJ, et al. Minimally invasive intrathoracic anastomosis after Ivor Lewis esophagectomy for cancer: a review of transoral or transthoracic use of staplers. Surg Endosc 2012;26:1795-802. [PubMed]
- Cadière GB, Dapri G, Himpens J, et al. Ivor Lewis esophagectomy with manual esogastric anastomosis by thoracoscopy in prone position and laparoscopy. Surg Endosc 2010;24:1482-5. [PubMed]
- Merritt RE. Initial experience of total thoracoscopic and laparoscopic Ivor Lewis esophagectomy. J Laparoendosc Adv Surg Tech A 2012;22:214-9. [PubMed]
- Pham TH, Perry KA, Dolan JP, et al. Comparison of perioperative outcomes after combined thoracoscopic-laparoscopic esophagectomy and open Ivor-Lewis esophagectomy. Am J Surg 2010;199:594-8. [PubMed]
- Sihag S, Wright CD, Wain JC, et al. Comparison of perioperative outcomes following open versus minimally invasive Ivor Lewis oesophagectomy at a single, high-volume centre. Eur J Cardiothorac Surg 2012;42:430-7. [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [PubMed]
- Noble F, Kelly JJ, Bailey IS, et al. A prospective comparison of totally minimally invasive versus open Ivor Lewis esophagectomy. Dis Esophagus 2013;26:263-71. [PubMed]
- Watson TJ. Robotic esophagectomy: is it an advance and what is the future? Ann Thorac Surg 2008;85:S757-9. [PubMed]
- Gharagozloo F, Margolis M, Tempesta BJ, et al. Robot-assisted ivor lewis esophagectomy for esophageal cancer. Chest 2007;132:659c-60.
- de la Fuente SG, Weber J, Hoffe SE, et al. Initial experience from a large referral center with robotic-assisted Ivor Lewis esophagogastrectomy for oncologic purposes. Surg Endosc 2013;27:3339-47. [PubMed]
- Cerfolio RJ, Bryant AS, Hawn MT. Technical aspects and early results of robotic esophagectomy with chest anastomosis. J Thorac Cardiovasc Surg 2013;145:90-6. [PubMed]